- sodium carbonate
-

- $1.00 / 1kg
-
2025-03-31
- CAS:497-19-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 mt
- sodium carbonate
-

- $10.00 / 1kg
-
2025-03-31
- CAS:497-19-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Sodium carbonate
-
- $0.00 / 200kg
-
2025-03-27
- CAS:497-19-8
- Min. Order: 20kg
- Purity: 99%
- Supply Ability: 20 tons
Related articles - Uses of Sodium carbonate
- Sodium Carbonate is the disodium salt of carbonic acid with alkalinizing property. When dissolved in water, sodium carbonate f....
- Feb 7,2022
Question and answer - Q:What is soda ash used for?
- A:Over half of all Soda Ash production is used in glass manufacturing, but it is used in the soap industry, paper production, p....
- Mar 13,2024
|
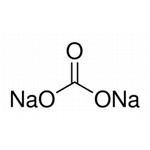
| Product Name: | Sodium carbonate | | Synonyms: | Sodium carbonate-12C, 13C-depleted;ODA ASH;SODA ASH LIGHT & SODA BI CARBONATE;SODA ASH (LIGHT AND DENSE );sodium carbonate, acs primary standard;sodium carbonate, anhydrous, puratronic;SODIUMCARBONATE,ANHYDROUS,BIOTECHGRADE;SODIUMCARBONATE,ANHYDROUS,GRANULAR,REAGENTSPECIAL,ACS | | CAS: | 497-19-8 | | MF: | CH2O3.2Na | | MW: | 105.99 | | EINECS: | 207-838-8 | | Product Categories: | Chemical Synthesis;S - ZTitration;Salt Solutions;Solution containers (VOLPAC);Synthetic Reagents;Chemical Synthesis;Inorganic Bases;Materials Science;Metal and Ceramic Science;Salts;Sodium Salts;Synthetic Reagents;metal carbonate;Alphabetical Listings;Q-S;Stable Isotopes;Inorganic BasesVolumetric Solutions;Chromatography/CE Reagents;Eluent concentrates for ICAlphabetic;Ion Chromatography;S;SN - SZ;By Reference Material;Concentrates (e.g. FIXANAL);Reference Material Hydrochloric acidTitration;Salt Concentrates;Inorganics;497-19-8 | | Mol File: | 497-19-8.mol | 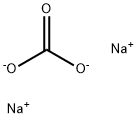 |
| | Sodium carbonate Chemical Properties |
| Melting point | 851 °C (lit.) | | Boiling point | 1600°C | | density | 2.53 | | bulk density | 1100kg/m3 | | refractive index | 1.535 | | storage temp. | 15-25°C | | solubility | H2O: 1 M at 20 °C, clear, colorless | | form | Solid | | color | White | | Specific Gravity | 2.532 | | pka | (1) 6.37, (2) 10.25 (carbonic (at 25℃) | | Odor | at 100.00?%. odorless | | Flame Color | Yellow | | PH | 10.52(1 mM solution);10.97(10 mM solution);11.26(100 mM solution); | | Water Solubility | 22 g/100 mL (20 ºC) | | Sensitive | Hygroscopic | | λmax | λ: 260 nm Amax: 0.01
λ: 280 nm Amax: 0.01 | | Merck | 14,8596 | | BRN | 4154566 | | Dielectric constant | 5.3(Ambient) | | Stability: | Stable. Incompatible with powdered alkaline earth metals, aluminium, organic nitro compounds, fluorine, alkali metals, nonmetallic oxides, concentrated sulfuric acid, oxides of phosphorus. | | InChIKey | CDBYLPFSWZWCQE-UHFFFAOYSA-L | | CAS DataBase Reference | 497-19-8(CAS DataBase Reference) | | NIST Chemistry Reference | Sodium carbonate(497-19-8) | | EPA Substance Registry System | Sodium carbonate (497-19-8) |
| | Sodium carbonate Usage And Synthesis |
| Description |
Sodium carbonate( 497-19-8), Na2CO3, is a sodium salt of carbonic acid. The pure product appears as a while, odorless powder with a strong alkaline taste. It has high hygroscopicity. It can be easily dissolved in water to form an aqueous solution with moderate alkalinity.
Sodium carbonate has wide applications in various kinds of fields around the world. One of most important application of sodium carbonate is for the manufacturing of glass. Based on statistics information, about half of the total production of sodium carbonate is used for the manufacturing of glass. During the production of glass, sodium carbonate acts as a flux in the melting of silica. In addition, as a strong chemical base, it is used in the manufacturing of pulp and paper, textiles, drinking water, soaps and detergents and as a drain cleaner. In addition, it can also be used for tissue digestion, dissolving amphoteric metals and compounds, food preparation as well as acting as a cleaning agent.
There are generally two ways for the production of sodium carbonate. One is through the reactions between sodium chloride and calcium carbonate (via the ammonia soda (Solvay) process). The other is from sodium carbonate and hydrogencarbonate ores (trona and nahcolite).
| | Physical Properties | Sodium carbonate is an inorganic salt and therefore the vapour pressure can be considered negligible. It has a melting point of 851°C (CRC Handbook, 1986; The Merck Index, 1983), it decomposes when heated at > 400 °C and therefore a boiling point cannot be determined. soluble in water; insoluble in alcohol; dissolves in acids liberating CO2.The octanol water partition coefficient (log Pow) is not relevant for an inorganic substance which dissociates. The average particle size diameter (d50) of light sodium carbonate is in the range of 90 to 150 µm and of dense sodium carbonate is in the range of 250 to 500 µm.

The monohydrate consists of colorless and odorless small crystals or cystalline powder; orthorhombic structure; refractive index 1.420; hardness 1.3 Mohs; density 2.25 g/cm3; loses water at 100°C becoming anhydrous; very soluble in water; insoluble in ethanol.
The decahydrate consists of transparent crystals; effloresces on exposure to air; density 1.46 g/cm3; decomposes at 34°C; very soluble in water; insoluble in ethanol.
An aqueous solution of sodium carbonate are strongly alkaline. | | Chemical Properties | Sodium carbonate is a white, crystalline and hygroscopic powder with a purity of > 98 %. There are two forms of sodium carbonate available, light soda and dense soda. Impurities of sodium carbonate may include water (< 1.5 %), sodium chloride (< 0.5 %), sulphate (< 0.1 %), calcium (< 0.1 %), magnesium (< 0.1 %) and iron (< 0.004 %). The purity and the impurity profile depends on the composition of the raw materials, the production process and the intended use of the product. For example the purity of the pharmaceutical grade must be higher than 99.5 % in Europe.
Sodium carbonate is a strong alkaline compound with a pH of 11.6 for a 0.1M aqueous solution (The Merck Index, 1983; Johnson and Swanson, 1987). The pKa of CO3 2- is 10.33, which means that at a pH of 10.33 both carbonate and bicarbonate are present in equal amounts.
| | Uses |
Sodium carbonate(497-19-8) is a kind of important raw material for chemical industry with wide application. It is the important raw material for making glass, soaps, detergents, textiles, leather, spices, dyes, medicines, etc.
Sodium carbonate was used as a pH modifier in toning baths and as the primary alkali in developers used for gelatin emulsions.
As the detergent, it can be used for wool rinse. It can also be applied to bath salts and pharmaceutical use and also be used as the alkali agent of tanning.
Sodium carbonate is most used in industry with a small part using by the civilian. In the soda ash of industry purpose, it is mainly applied to light industry, building materials and chemical industry, accounting for about 2/3: followed by metallurgy, textiles, petroleum, defense, and pharmaceutical. The glass industry is the largest soda consumer sector with each ton of glass consuming 0.2 ton of soda ash. In the chemical industry, it can be used for manufacturing of sodium silicate, sodium dichromate, sodium nitrate, sodium fluoride, baking soda, borax, and trisodium phosphate. In the metallurgical industry, it is mainly used for fluxing agent, mineral flotation agent, and desulfurization agent for steel and antimony. It can also be used as water softener in printing and dyeing industry. In tanning industry, it can be used for the degreasing of raw hides, neutralizing chrome tanned leather and improving the alkalinity of the chrome liquid. It is also used in the production of synthetic detergent additive sodium tripolyphosphate and other sodium salt.
| | Biological Functions |
Sodium carbonate(497-19-8) is used as a buffer component in such applications as chromatography, capillary electrophoresis, and enzyme catalysis. Sodium carbonate is widely used in the isolation of cell membranes, membrane proteins, and hydrophobic proteins. A protocol for the isolation of polyamines from cell culture media has been published.
| | Toxicity | ADI (acceptable daily intake) make no restrictions (FAO/WHO in 1985). LD50 (median lethal dose) is about 6 g/kg (mice-oral).
Soda ash dust has irritation effects on the skin, respiratory and eyes. Long-term exposure to soda solution may cause eczema and dermatitis. Its concentrated solutions can cause burns, necrosis, and even corneal opacity. The maximal allowable concentration of soda ash dust in the air is 2 mg/m3. The operators should wear overalls, door cover, gloves, boots and other protective clothing to protect the respiratory system and skin.
| | Production method | Sodium carbonate at present is mostly mined from its natural deposits. It also is manufactured syntheticallly by Solvay (or ammonia-soda) process. The natural production of sodium carbonate currently has supassed its synthetic production.
The Solvay process involves a series of partial reactions. The first step is calcination of calcium carbonate to form lime and CO2. Lime is converted to calcium hydroxide. The most crucial step of the process involves reacting brine solution with carbon dioxide and ammonia to produce sodium bicarbonate and ammonium chloride. Sodium bicarbonate converts to sodium carbonate. The calcium hydroxide and ammonium chloride react to form calcium chloride as the by-product. The partial reactions are shown below:
CaCO3 → CaO + CO2
CaO + H2O → Ca(OH)2
2NaCl + 2CO2 + 2NH3 + 2H2O → 2NaHCO3 + 2NH4Cl
2NaHCO3 → Na2CO3 + H2O + CO2
Ca(OH)2 + 2NH4Cl → CaCl2 + 2NH3 + 2H2O
The overall reaction:
CaCO3 + 2NaCl → Na2CO3 + CaCl2
Sodium carbonate was made historically by the Leblanc process. The first commercial production was carried out by the Leblanc process. In this process, sodium chloride was treated with sulfuric acid to produce sodium sulfate and hydrochloric acid. Heating the sodium sulfate with coal and limestone produced a “black ash” that contained sodium carbonate, calcium sulfide, unreacted coal, and calcium carbonate. Sodium carbonate was separated from the black ash by leaching with water. The overall reaction is as follows:
Na2SO4 + 2C + CaCO3 → Na2CO3 + CaS + 2CO2
| | References | https://en.wikipedia.org/wiki/Sodium_hydroxide#Uses
http://www.essentialchemicalindustry.org/chemicals/sodium-carbonate.html | | Description | Sodium carbonate is known as soda ash or washing soda and is a heavily used inorganic compound. Approximately 45 million tons of soda ash are produced globally both naturally and synthetically. Soda ash is obtained naturally primarily from the mineral trona, but it can also be obtained from nahcolite (NaHCO3) and salt brine deposits. Trona is a freshwater sodium carbonate-bicarbonate evaporite, with the formula Na3CO3HCO3 .2H2O. The largest known deposit of trona is located in the Green River area of Wyoming, and other large deposits are found in Egypt’s Nile Valley and California’s Searles basin around the city of Trona. Soda ash is produced from mined trona by crushing and screening the ore and then heating it. Th is produces a soda ash mixed with impurities. Pure soda ash is obtained by dissolving the product and precipitating impurities combined with filtering processes. | | Chemical Properties | Sodium carbonate, Na2C03, also known as soda or soda ash,is the most important of the industrial alkalis. It is a white or grayish-white, lumpy, water-soluble powder that loses its water of crystallization when heated. It decomposes at a temperature of about 852°C (1560°F). It exists in solution only. It is prepared by the combination of carbon dioxide and water. | | Physical properties | Anhydrous sodium carbonate (soda ash, sal soda) is a white powder, which cakes and aggregates on exposure to air due to the formation of hydrates. The monohydrate, Na2CO3·H2O, is a white crystalline material, which is soluble in water and insoluble in alcohol; r.d. 2.532; loses water at 109°C; m.p. 851°C. The decahydrate, Na2CO3·10H2O (washing soda), is a translucent efluorescent crystalline solid; r.d. 1.44; loses water at 32–34°C to give the monohydrate; m.p. 851°C. | | Occurrence | Ash is a tree found in regions of North America | | History | Sodium carbonate, Na2CO3, has been used historically for making glass, soap, and gunpowder. Along with potassium carbonate, known as potash, sodium carbonate was the basis of the alkali industry, which was one of the first major chemical industries. Throughout history, alkalis were obtained from natural sources. Soda ash was also produced by burning wood and leaching the ashes with water to obtain a solution that yielded soda ash when the water was boiled off. The name soda ash originates from the barilla plant, which was used to produce soda ash. The scientific name of this plant is Salsola soda, but it goes by the common names of sodawort or glasswort because the soda produced from it was used in making glass. Barilla is a common plant found in saline waters along the Mediterranean Sea in Spain and Italy.
Barilla was dried and burned to produce soda ash. The depletion of European forests and
international disputes made the availability of alkali salts increasingly uncertain during the
latter part of the 18th century. LeBlanc
proposed a procedure in 1783, and a plant based on LeBlanc’s method was opened in 1791.
Unfortunately, LeBlanc’s association with French Royalty led to the confi scation of the plant
at the time of the French Revolution. Furthermore, confl icting claims for LeBlanc’s method
were made by several other chemists and he never received the reward. | | Uses | Sodium Carbonate is an alkali that exists as crystals or crystalline
powder and is readily soluble in water. it has numerous functions:
an antioxidant, a curing and pickling agent, a flavoring agent, a
processing aid, a sequestrant, and an agent for ph control. it is used
in instant soups to neutralize acidity. it is used in alginate water des-
sert gels to sequester the calcium, allowing the alginate to solubilize.
it is also used in puddings, sauces, and baked goods. | | Uses | Soda ash is used in glass making, in production of sodium chemicals (such as sodium chromates, phosphates, and silicates), in the wood pulp industry, in production of soaps and detergents, in oil refining, in water softening, and in refining of nonferrous metals. In its hydrous crystallized form (Na2C03.10H2O), it is known as sal soda,washing soda,or soda crystals, not to be confused with baking soda,which is sodium hydrogen carbonate or sodium bicarbonate (NaHC03). Its monohydrate form(Na2C03·H20) is the standard compound for scouring solutions.
When in solution, sodium carbonate creates less alkalinity than the hydroxides. A 0.1% solution creates a pH of 11;a fully saturated solution is 35%, which has a pH of 12.5.
The safety requirements for sodium carbonate, because of its lower alkalinity, can be considered less demanding than those for the related bicarbonates. | | Production Methods | Sodium carbonate is produced on all continents of the world
from its minerals. It is present in large deposits in Africa and the United States as either carbonate or trona, a mixed ore of
equal molar amounts of carbonate and bicarbonate. However,
about 70% of the world production of sodium carbonate is
manufactured by the Solvay (ammonia soda) process,
whereby ammonia is added to a solution of sodium chloride.
Carbon dioxide is then bubbled through to precipitate the
bicarbonate (NaHCO3) that is decomposed by heat-producing
sodium carbonate. In the United States. all production is
based on the minerals that contain sodium carbonate. Different
qualities of sodium carbonate are produced: technical,
food, and pharmaceutical grades. | | Definition | Sodium carbonate is an organic sodium salt and a carbonate salt. A dibasic acid formed in small amounts in solution when carbon dioxide dissolves in water: CO2 + H2O=H2CO2It forms two series of salts: hydrogencarbonates (HCO3–) and carbonates (CO32-). The pure acid cannot be isolated. | | General Description | Sodium carbonate is a water soluble inorganic salt commonly used as a weak base. Its aqueous solution has the ability to uptake carbon dioxide. It can also catalyze the conversion of sewage sludge to liquid fuels. | | Flammability and Explosibility | Non flammable | | reaction suitability | reaction type: Acid-base reactions | | Biochem/physiol Actions | Buffer component, may be used for the removal of peripheral membrane proteins. | | Safety Profile | Poison by intraperitoneal route. Moderately toxic by inhalation and subcutaneous routes. Mlldly toxic by ingestion. Experimental reproductive effects. A skin and eye irritant. It migrates to food from packagmg materials. Can react violently with Al, P2O5, H2SO4, F2, Li, 2,4,6-trinitrotoluene. When heated to decomposition it emits toxic fumes of Na2O | | Purification Methods | It crystallises from water as the decahydrate which is redissolved in water to give a near-saturated solution. By bubbling CO2, NaHCO3 is precipitated. It is filtered off, washed and ignited for 2hours at 280o [MacLaren & Swinehart J Am Chem Soc 73 1822 1951]. Before being used as a volumetric standard, analytical grade material should be dried by heating at 260-270o for 0.5hour and allowed to cool in a desiccator. It has a transition point at 450o, and its solubility in water is 21.58% at 20o (decahydrate in solid phase), 49.25% at 35o (heptahydrate in solid phase) and 44.88% at 75o(monohydrate in solid phase) [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 987-988 1963]. After three recrystallisations, technical grade Na2CO3 had Cr, Mg, K, P, Al, W, Sc and Ti at 32, 9.4, 6.6, 3.6, 2.4, 0.6, 0.2 and 0.2 ppm respectively; another technical source had Cr, Mg, Mo, P, Si, Sn and Ti at 2.6, 0.4, 4.2, 13.4, 32, 0.6, 0.8 ppm respectively. |
| | Sodium carbonate Preparation Products And Raw materials |
|