- Amrubicin
-

- $15.00 / 1KG
-
2021-07-02
- CAS:110267-81-7
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- AMRUBICIN
-

- $1.00 / 1g
-
2019-12-26
- CAS:110267-81-7
- Min. Order: 1g
- Purity: ≥98%
- Supply Ability: g/kg/Ton
|
| | Amrubicin Basic information |
| Product Name: | Amrubicin | | Synonyms: | (7S,9S)-9-Acetyl-9-amino-7-[(2-deoxy--D-erythro-pentopyranosyl)oxy]-7,8,9,10-tetrahydro-6,11-dihydroxy-5,12-naphthacenedione;(7S)-9β-Amino-9-acetyl-7β-[(2-deoxy-β-D-erythro-pentopyranosyl)oxy]-7,8,9,10-tetrahydro-6,11-dihydroxynaphthacene-5,12-dione;(7S,9S)-9-Acetyl-9-amino-7-(2-deoxy-β-D-erythro-pentopyranosyloxy)-6,11-dihydroxy-5,7,8,9,10,12-hexahydro-5,12-naphthacenedione;(7S,9S)-9-Amino-9-acetyl-7-[(2-deoxy-β-D-erythro-pentopyranosyl)oxy]-7,8,9,10-tetrahydro-6,11-dihydroxynaphthacene-5,12-dione;Amurubicin;5,12-Naphthacenedione,9-acetyl-9-aMino-7-(2-deoxy-b-D-erythro-pentopyranosyl)oxy-7,8,9,10-tetrahydro-6,11-dihydroxy-,(7S,9S)-;CS-1168;5,12-Naphthacenedione, 9-acetyl-9-amino-7-[(2-deoxy-β-D-erythro-pentopyranosyl)oxy]-7,8,9,10-tetrahydro-6,11-dihydroxy-, (7S,9S)- | | CAS: | 110267-81-7 | | MF: | C25H25NO9 | | MW: | 483.47 | | EINECS: | | | Product Categories: | Carbohydrates & Derivatives;Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 110267-81-7.mol | 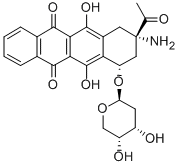 |
| | Amrubicin Chemical Properties |
| Melting point | 172-1740C | | alpha | D20 +119° (c = 0.02 in CHCl3) | | Boiling point | 717.8±60.0 °C(Predicted) | | density | 1.60±0.1 g/cm3(Predicted) | | storage temp. | Store at -20°C | | solubility | >16.45mg/mL in DMSO | | form | Powder | | pka | 7.83±0.60(Predicted) | | color | Pink to red |
| | Amrubicin Usage And Synthesis |
| Description | Amrubicin is an active agent for the treatment of small-cell lung cancer (SCLC). It is a type of anthracycline analog. Anthracycline derivatives such as doxorubicin (DXR), daunorubicin (DNR) and epirubicin have been widely used for a variety of carcinomas in the clinical context. Amrubicin is structurally distinguishable from other anthracyclines by the amino group at the 9-position and its unique sugar moiety. In April 2002, Amrubicin was approved in Japan for the treatment of non-small cell lung cancer and small cell lung cancer. | | Uses | Synthetic anthracycline antibiotic; inhibits DNA topoisomerase II. Antineoplastic. Amrubicin is used to treat small-cell lung cancer (SCLC). | | Definition | ChEBI: Amrubicin is a synthetic anthracycline antibiotic with molecular formula C25H25NO9. A specific inhibitor of topoisomerase II, it is used (particularly as the hydrochloride salt) in the treatment of cancer, especially lung cancer, where it is a prodrug for the active metabolite, ambrucinol. It has a role as a topoisomerase II inhibitor, an antineoplastic agent and a prodrug. It is a quinone, a member of tetracenes, a methyl ketone, an anthracycline antibiotic and a primary amino compound. | | target | Topoisomerase II | | Mode of action | Amrubicin (AMR), a third-generation anthracycline and potent topoisomerase II inhibitor that intercalates into DNA and inhibits the activity of topoisomerase II, resulting in inhibition of DNA replication, and RNA and protein synthesis, followed by cell growth inhibition and cell death. Amrubicin is structurally distinguishable from other anthracyclines by the amino group at the 9-position and its unique sugar moiety. This agent has demonstrated a higher level of anti-tumor activity than conventional anthracycline drugs without exhibiting any indication of the cumulative cardiac toxicity common to this class of compounds. |
| | Amrubicin Preparation Products And Raw materials |
|