|
|
| | PARAFORMALDEHYDE Basic information |
| Product Name: | PARAFORMALDEHYDE | | Synonyms: | PARAFORM;POLYACETAL;POLYACETAL RESIN;POLYFORMALDEHYDE;POLY(OXYMETHYLENE), ACETATE END-CAPPED;POLYOXMETHYLENE;POLY(TRIOXANE);FORMALDEHYDE RESIN | | CAS: | 25231-38-3 | | MF: | C3H6O3X2 | | MW: | 90.08 | | EINECS: | 607-656-2 | | Product Categories: | Polymers | | Mol File: | 25231-38-3.mol |  |
| | PARAFORMALDEHYDE Chemical Properties |
| Hazard Codes | Xn | | Risk Statements | 31 | | Safety Statements | 36 | | RIDADR | UN 2213 4.1/PG 3 | | WGK Germany | 3 | | RTECS | RV0540000 |
| | PARAFORMALDEHYDE Usage And Synthesis |
| Chemical Properties | The homopolymers and copolymers of formaldehyde, prepared as described
above, are rigid materials with broadly similar properties. They are particularly noted for their stiffness, fatigue resistance and creep resistance and are
counted as one of the 'engineering plastics'. They find application principally
in injection moulded mechanical parts such as gears, cams and plumbing
components. The copolymers are somewhat less crystalline
and therefore have lower density, melting point, hardness, tensile strength
and flexural modulus. The main advantage claimed for the copolymers is
improved processability, with less degradation at processing temperatures.
As is characteristic of crystalline polymers which do not interact with any
liquids, there are no effective solvents at room temperature for the commercial formaldehyde polymers. At temperatures above 70°C, solution occurs in
a few solvents such as the chlorophenols. The resistance of the polymers to
inorganic reagents is not, however, outstanding. Strong acids, strong alkalis
and oxidizing agents cause a deterioration in mechanical properties. (The
copolymers are significantly superior to the homopolymers in alkali resistance.)
Oxidation of polyformaldehyde occurs in air on prolonged exposure to
ultraviolet light and/or elevated temperature. Antioxidants are therefore
commonly added to the polymers. | | Preparation | (a) Homopolymers
In the preparation of high molecular weight polyformaldehyde the initial
operation consists of the production of pure formaldehyde, free from low molecular weight polymers and other hydroxy compounds which cause chain
transfer. In a typical process potassium hydroxide-precipitated paraformal�dehyde (degree of polymerization approximately 200) is carefully washed
with water and dried for several hours in vacuo at 80??C. The dried polymer is
then decomposed in nitrogen at 150-160??C; the product is passed through
several traps at -15??C to remove water, glycols, and other impurities. The
resulting formaldehyde has a water content (free and combined) of less
than 0.1 %.
The formaldehyde is then introduced into a reactor where it passes over the
surface of a rapidly stirred solution of initiator (either a Lewis acid or base;
triphenylphosphine appears to be favoured) in a carefully dried inert medium
such as heptane at about 40??C. The process is designed to give a very low
concentration of formaldehyde to minimize transfer from polymer to
monomer. To the initiator solution may be added a polymer stabilizer (e.g.
diphenylamine) and transfer agents (e.g. traces of water or methanol). Polymerization is continued until the concentration of polymer in the slurry is
about 20% and then the polymer is collected by filtration.
In the final stage the polymer is subjected to an esterification reaction to
improve its thermal stability. The esterification may be effected with a
number of anhydrides, but acetic anhydride is generally preferred. Typically,
the polyformaldehyde is heated under slight pressure to about 160??C with
acetic anhydride and a small amount of sodium acetate (catalyst). The
polymer is soluble in acetic anhydride at this temperature but is precipitated
when the solution is cooled. The acetylated polymer is collected by filtration,
washed with water (to remove the anhydride and catalyst) and then acetone
(containing di-fi-naphthyl-p-phenylenediamine as antioxidant), and dried in
vacuo at 70??C. The product is then extruded and chopped into granules. The
average molecular weight (Mn) of the polyformaldehyde produced by these
methods is generally in the range 30000-100000.
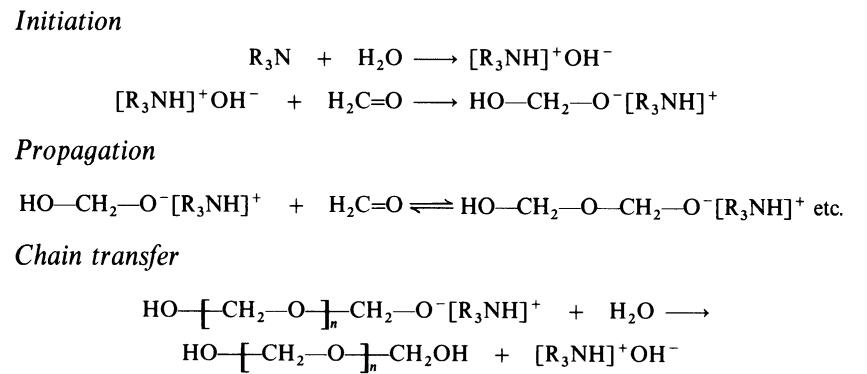
The polymerization of formaldehyde by Lewis bases such as triaryl amines
(R3N), arsines, and phosphines proceeds by the following anionic mechanism:
The polymerization of formaldehyde by Lewis acids such as boron trifluor�ide proceeds according to following cationic mechanism:
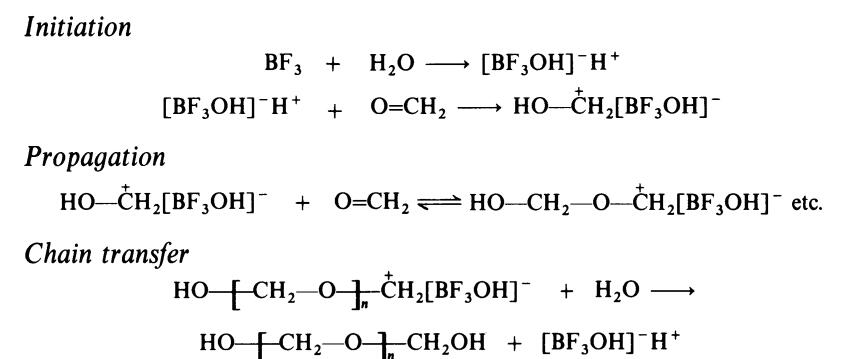
The hydroxy-terminated polymers have poor thermal stability. Loss of a
proton, possibly to an initiator residue, from a chain end gives an anion
capable of decomposing to formaldehyde by the reverse of the propagation
process. The stability of the polymer is therefore improved if the hydroxy end�groups are removed by esterification:
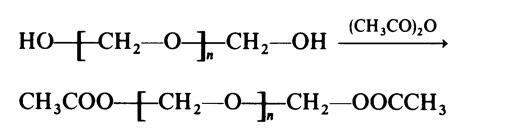
It may be noted here that the polymerization of formaldehyde cannot be
effected with free radical initiators.
(b) Copolymers
Details of the procedures used in the preparation of commercial formalde�hyde copolymers have not been fully disclosed. The principal monomer is
trioxan and the second monomer is a cyclic ether such as ethylene oxide, 1,3-
dioxolane or an oxetane; ethylene oxide appears to be the preferred
comonomer and is used at a level of about 2%. Boron trifluoride (or its
etherate) is apparently the most satisfactory initiator, although many cationic
initiators are effective; anionic and free radical initiators are not effective. The
reaction is carried out in bulk. The rapid solidification of the polymer
requires a reactor fitted with a powerful stirrer to reduce particle size and
permit adequate temperature control. The copolymer is then heated at lOoDe
with aqueous ammonia; in this step, chain-ends are depolymerized to the
copolymer units to give a thermally-stable product. The polymer is filtered off
and dried prior to stabilizer incorporation, extrusion and granulation.
The mechanism of polymerization of trioxan has not been completely
elucidated. A possible scheme, in which boron trifluoride-water is the initiator is as follows:
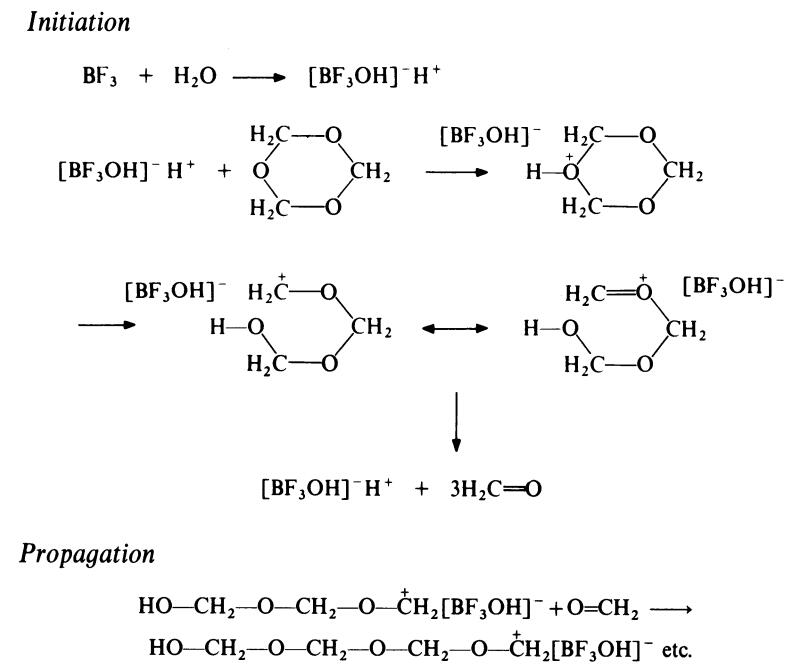
In the first step, trioxan is protonated by the complex protic acid formed by
interaction of boron trifluoride and water. (It has been shown that no
reaction occurs in the complete absence of water). The resulting oxonium
ion undergoes ring-opening to give a resonance-stabilized species. This then
depolymerizes to build up an equilibrium concentration of formaldehyde,
which remains constant during the polymerization. The actual propagation
step then involves the addition of formaldehyde rather than trioxan. This
scheme accounts for the observation that the polymerization of pure trioxan
involves an induction period which may be reduced or even eliminated by the
addition of formaldehyde. |
| | PARAFORMALDEHYDE Preparation Products And Raw materials |
|