|
|
| | (1R,2S)-2-(3,4-difluorophenyl)cyclopropanamine hydrochloride Basic information | | Physical Form |
| Product Name: | (1R,2S)-2-(3,4-difluorophenyl)cyclopropanamine hydrochloride | | Synonyms: | (1R,2S)-2-(3,4-Difluorophenyl)-cyclopropan-1-aMine HCL;(1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanami;2-(3,4-Difluoro-phenyl)-cyclopropylamine;(1R trans)-2-(3,4-difluorophenyl)cyclopropane amine;CYCLOPROPANAMINE, 2-(3,4-DIFLUOROPHENYL)-, HYDROCHLORIDE (1:1), (1R,2S)-REL-;trans-(1R,2S)-2-(3,4-difluorphenyl)cyclopropylaMine;(1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanamine HCl;(1R,2S)-rel-2-(3,4-Difluorophenyl) cyclopropanamine hydrochlorideQ: What is
(1R,2S)-rel-2-(3,4-Difluorophenyl) cyclopropanamine hydrochloride Q: What is the CAS Number of
(1R,2S)-rel-2-(3,4-Difluorophenyl) cyclopropanamine hydrochloride Q: What is the storage condition of
(1R,2S)-rel-2-(3,4-Difluorophenyl) cyclopropanamine hydrochloride | | CAS: | 1156491-10-9 | | MF: | C9H10ClF2N | | MW: | 205.63 | | EINECS: | 829-852-9 | | Product Categories: | Intermediate of Ticagrelor;1156491-10-9 | | Mol File: | 1156491-10-9.mol | 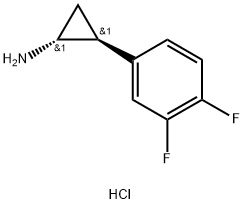 |
| | (1R,2S)-2-(3,4-difluorophenyl)cyclopropanamine hydrochloride Chemical Properties |
| Melting point | >172°C (dec.) | | storage temp. | Inert atmosphere,Store in freezer, under -20°C | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | color | White | | InChI | InChI=1/C9H9F2N.ClH/c10-7-2-1-5(3-8(7)11)6-4-9(6)12;/h1-3,6,9H,4,12H2;1H/t6-,9+;/s3 | | InChIKey | IMYLOCHFFLYHPS-RHUSADAZNA-N | | SMILES | N[C@@H]1C[C@H]1C1C=CC(F)=C(F)C=1.Cl |&1:1,3,r| |
| | (1R,2S)-2-(3,4-difluorophenyl)cyclopropanamine hydrochloride Usage And Synthesis |
| Physical Form | Solid | | Uses | (1S,2R)-2-(3,4-Difluorophenyl)-cyclopropanamine is an intermediate used to prepare ticagrelor (T437700). | | Synthesis Reference(s) | Synthesis of 2-(3,4-difluorophenyl)cyclopropanamine derivatives and salts. EU Patent EP2644590A1
| | Synthesis |  1,2-Difluorobenzene is reacted with 3-chloropropionyl chloride to
produce 3-chloro-1-(3',4'-difluorophenyl)-propan-1-one, which is by
addition of N,N-dimethylformamide,
phloroglucinol and sodium iodide in the next step converted to
1-(3',4'-difluorophenyl)-3-nitro-propan-1-one. The keto group of the
latter intermediate is in the subsequent step stereochemically reduced
to hydroxyl group by the use of chiral oxazaborolidine together with
borane dimethyl sulfide or borane-N,N-diethyl aniline complex in the presence of tetrahydrofurane. The obtained (1R)-1-(3',4'-difluorophenyl)-3-nitro-propan-1-ol
is then added to a mixture of triphenylphosphine and
diethylazodicarboxylate in benzene to yield (1S,2R)-2-(3',4'-difluorophenyl)-1-nitrocyclopropane, which is in the last step converted to 2-(3,4-difluorophenyl)cyclopropane amine by reduction of the nitro group by catalytic hydrogenation with palladium catalyst and zink dust. 1,2-Difluorobenzene is reacted with 3-chloropropionyl chloride to
produce 3-chloro-1-(3',4'-difluorophenyl)-propan-1-one, which is by
addition of N,N-dimethylformamide,
phloroglucinol and sodium iodide in the next step converted to
1-(3',4'-difluorophenyl)-3-nitro-propan-1-one. The keto group of the
latter intermediate is in the subsequent step stereochemically reduced
to hydroxyl group by the use of chiral oxazaborolidine together with
borane dimethyl sulfide or borane-N,N-diethyl aniline complex in the presence of tetrahydrofurane. The obtained (1R)-1-(3',4'-difluorophenyl)-3-nitro-propan-1-ol
is then added to a mixture of triphenylphosphine and
diethylazodicarboxylate in benzene to yield (1S,2R)-2-(3',4'-difluorophenyl)-1-nitrocyclopropane, which is in the last step converted to 2-(3,4-difluorophenyl)cyclopropane amine by reduction of the nitro group by catalytic hydrogenation with palladium catalyst and zink dust. |
| | (1R,2S)-2-(3,4-difluorophenyl)cyclopropanamine hydrochloride Preparation Products And Raw materials |
|