|
|
| | Diethyl allylphosphonate Basic information |
| | Diethyl allylphosphonate Chemical Properties |
| Boiling point | 46 °C/0.35 mmHg (lit.) | | density | 1,035 g/cm3 | | bulk density | 1.022g/mL | | vapor pressure | 55.2Pa at 25℃ | | refractive index | n20/D 1.4340(lit.) | | Fp | 224 °F | | form | Liquid | | color | Clear colorless | | InChI | InChI=1S/C7H15O3P/c1-4-7-11(8,9-5-2)10-6-3/h4H,1,5-7H2,2-3H3 | | InChIKey | YPJHXRAHMUKXAE-UHFFFAOYSA-N | | SMILES | P(CC=C)(=O)(OCC)OCC | | LogP | 1.33 at pH7 | | Surface tension | 56.4-60.9mN/m at 1g/L and 19℃ | | CAS DataBase Reference | 1067-87-4(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | HS Code | 29319090 |
| | Diethyl allylphosphonate Usage And Synthesis |
| Chemical Properties | clear colorless liquid | | Uses | Reactant for:
- Copolymerization of phosphonated allyl monomers and maleic anhydride
- Enantioselective synthesis of solamin type mono-THF acetogenins
- RCM reaction yielding oxaphospholene and oxaphosphinene heterocycles
- Synthesis of spongistatin 2 using Wittig coupling
- Stereoselective synthesis of pentacyclic furanosteroids
- Preparation of protected polyhydroxylated β -amino acid constitutents of microsclerodermins
| | Reactions | To a stirred solution of diethyl allylphosphonate (582.5 mg, 3.0 mmol, 1.5 equiv) in dry THF (25 mL) was added NaH (60% dispersion in mineral oil, 120 mg, 3.0 mmol, 1.5 equiv) portion wise under nitrogen atmosphere at 0°C. After 15 min, aryl/alkyl aldehyde 14a–x, 14z (2.0 mmol) in dry THF was added to the reaction mixture and stirred at 0°C until the completion (monitored by TLC) of reaction. It was then quenched with saturated aq. NH4Cl and the solution was extracted with EtOAc (2×20 mL). The combined organic layers were dried (Na2SO4) and concentrated. The residue was purified by silica gel column chromatography using petroleum ether/EtOAc (9:1, common for all compounds unless specified) as eluent to afford (E)-1-aryl/alkylbutadienes 7a–x, 7z.
The CM reaction of 2 with dimethyl vinylphosphonate (3a) and diethyl allylphosphonate (3b) under a classic thermal condition with 3 mol % of H-G II at 40 °C.
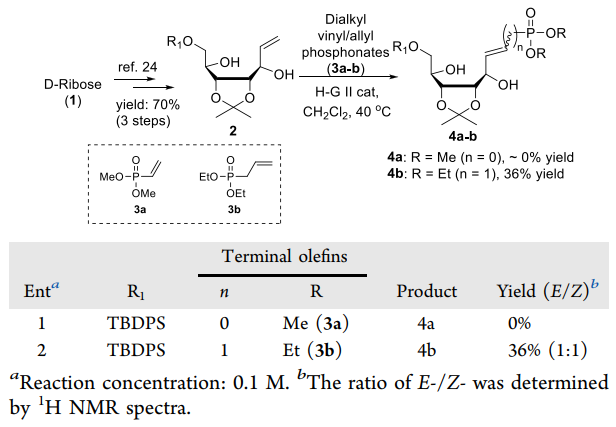 | | Synthesis Reference(s) | Synthetic Communications, 22, p. 2219, 1992 DOI: 10.1080/00397919208019075
Synthesis, p. 563, 1986 DOI: 10.1055/s-1986-31704 | | reaction suitability | reaction type: C-C Bond Formation | | References | [1] CHOUDHARY P, RANJAN R S, FERNANDES R. Cu-Catalyzed Coupling of Sulfonyl Chlorides with Alkenes: Synthesis of Dienyl Sulfones and β-Chlorosulfones[J]. European Journal of Organic Chemistry, 2024, 28 1. DOI:10.1002/ejoc.202401077.
[2] SE MYEONG CHOI, JONG HYUN CHO* Direct Synthesis of Aryloxy Phosphonamidate Nucleotide Prodrugs Using the Cross Metathesis Assisted by Ultrasonic Irradiation[J]. Organic Letters, 2024, 26 23: 4841-4846. DOI:10.1021/acs.orglett.4c00094.
[3] ELIZABETH I. PARKINSON. Fosmidomycin biosynthesis diverges from related phosphonate natural products[J]. Nature chemical biology, 2019, 15 11: 1049-1056. DOI:10.1038/s41589-019-0343-1.
[4] PAWE? MITU?A C. W. Synthesis of a series of new racemic [2,3-bis(acyloxy)propyl]phosphonocholines[J]. Arkivoc, 2012, 2012 1. DOI:10.3998/ARK.5550190.0013.416. |
| | Diethyl allylphosphonate Preparation Products And Raw materials |
|