|
|
| Product Name: | Tin(IV) chloride | | Synonyms: | TIN(IV) CHLORIDE 5H2O;TIN(IV) CHLORIDE-5-HYDRATE;TIN TETRACHLORIDE PENTAHYDRATE;STANNIC CHLORIDE PENTAHYDRATE;STANNIC CHLORIDE 5H2O;STANNIC CHLORIDE, 5-HYDRATE;Stannane,tetrachloro-,pentahydrate;stannicchloride,crystal | | CAS: | 10026-06-9 | | MF: | Cl4H2OSn | | MW: | 278.53 | | EINECS: | 600-048-8 | | Product Categories: | metal halide | | Mol File: | 10026-06-9.mol | 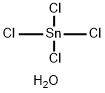 |
| | Tin(IV) chloride Chemical Properties |
| Melting point | 56 °C | | density | 2.040 | | solubility | very soluble in H2O; soluble in ethanol | | form | lumps | | color | Light Yellow | | Water Solubility | soluble | | Merck | 14,8774 | | Stability: | Stable, but may decompose in contact with moisture or water. Incompatible with strong acids. | | InChIKey | KHMOASUYFVRATF-UHFFFAOYSA-J | | CAS DataBase Reference | 10026-06-9(CAS DataBase Reference) | | EPA Substance Registry System | Stannane, tetrachloro-, pentahydrate (10026-06-9) |
| Hazard Codes | C | | Risk Statements | 34-52/53 | | Safety Statements | 26-36/37/39-45-7/8-61 | | RIDADR | UN 2440 8/PG 3 | | WGK Germany | 3 | | RTECS | XP8870000 | | HazardClass | 8 | | PackingGroup | III | | HS Code | 28273990 |
| | Tin(IV) chloride Usage And Synthesis |
| Physical Properties | Colorless fuming liquid; corrosive; density 2.234 g/mL; freezes at –33°C; boils at 114.15°C; critical temperature 318.75°C; critical pressure 37.98 atm; critical volume 351 cm3/mol; soluble in cold water, evolving heat; decomposed by hot water; soluble in alcohol, benzene, toluene, chloroform, acetone and kerosene.
The pentahydrate is a yellowish-white crystalline solid or small, fused lumps; faint odor of HCl; density 2.04 g/cm3; decmposes at 56°C; very soluble in water; soluble in ethanol.
| | Uses | Tin(IV) chloride is a mordant for dying fabrics; a stabilizer for perfume in soap; used in weighting silk; in ceramic coatings; in manufacturing blue print papers; and to produce fuchsin. Also, tin(IV) chloride is used in preparing many organotin compounds.
| | Preparation | Tin(IV) chloride is prepared by reacting tin or tin(II) chloride with chlorine:
Sn + 2Cl2 → SnCl4
SnCl2 + Cl2 → SnCl4
| | Chemical Properties | White solid. Soluble in water or alcohol. | | Chemical Properties | Stannic chloride is a white to yellow powder with a faint odor of HCl. | | Uses | Tin (IV) Chloride Pentahydrate is a hydrate of Tin (IV) Chloride(T443805), which can induce slight inhibition of the catalytic activity of horse liver alcohol dehydrogenase (HLADH). | | Uses | Substitute for anhydrous stannic chloride where the presence of water is not objectionable | | General Description | Stannic chloride pentahydrate is a white colored solid. Stannic chloride pentahydrate is soluble in water. Stannic chloride pentahydrate is toxic by ingestion, inhalation and skin absorption. Stannic chloride pentahydrate is used to make perfumes and dyes. | | Air & Water Reactions | Soluble in water. Reacts with water to form Hydrochloric Acid in dense white fumes [Merck 11th ed. 1989]. | | Reactivity Profile | Acidic salts, such as STANNIC CHLORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. | | Hazard | Toxic material | | Health Hazard | TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | | Fire Hazard | Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. | | reaction suitability | reagent type: catalyst
core: tin | | Safety Profile | A poison by
intraperitoneal and intravenous routes.
Mutation data reported. A corrosive liquid.
When heated to decomposition it emits
toxic vapors of tin and Cl-. | | Potential Exposure | Hydrated stannic chloride is used for fixing certain textile dyes, and for treating silk to give weight to the fabric. | | Shipping | UN2440 Stannic chloride, pentahydrate, Hazard class: 8; Labels: 8-Corrosive material. | | Incompatibilities | Reacts violently with water, forming corrosive hydrochloric acid and tin oxide fumes. Reacts with turpentine, alcohols, and amines, causing fire and explosion hazard. Attacks many metals; some forms of plastics, rubber, and coatings. Reacts with moist air to form hydrochloric acid and dense white fume. |
| | Tin(IV) chloride Preparation Products And Raw materials |
|