| Company Name: |
3B Pharmachem (Wuhan) International Co.,Ltd.
|
| Tel: |
821-50328103-801 18930552037 |
| Email: |
3bsc@sina.com |
| Products Intro: |
Product Name:ALAMETHICIN
CAS:59588-86-2
Purity:99% HPLC Package:1Mg ; 5Mg;10Mg ;100Mg;250Mg ;500Mg ;1g;2.5g ;5g ;10g
|
| Company Name: |
Creative Peptides
|
| Tel: |
|
| Email: |
info@creative-peptides.com |
| Products Intro: |
Product Name:Alamethicin
CAS:59588-86-2
Purity:>98% Package:1g; 10g;100g;1kg Remarks:Antimicrobial Peptides
|
| Company Name: |
Hunan chemfish Scientific co.,ltd
|
| Tel: |
+8618229978283 19186994443 |
| Email: |
sales@chemfish.com |
| Products Intro: |
Product Name:ALAMETHICIN
CAS:59588-86-2
Purity:95% Package:100G,1KG,10KG,100KG
|
|
| | ALAMETHICIN Basic information |
| Product Name: | ALAMETHICIN | | Synonyms: | alamethicinf30;alamethicingamma;alamethicini;f-50;U-22324;ANTIBIOTIC U-22324;ALMETHICIN;ALAMETHACIN | | CAS: | 59588-86-2 | | MF: | C92H150N22O25 | | MW: | 1964.31 | | EINECS: | | | Product Categories: | | | Mol File: | 59588-86-2.mol | 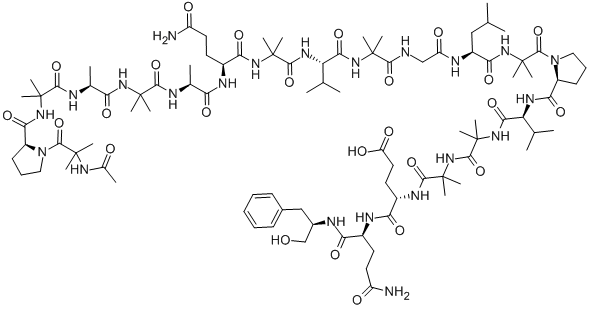 |
| | ALAMETHICIN Chemical Properties |
| Melting point | 267-270℃ | | RTECS | AY1900000 | | storage temp. | 2-8°C | | solubility | Soluble in DMSO and ethanol. | | form | Powder |
| Hazard Codes | T | | Risk Statements | 25 | | Safety Statements | 45 | | RIDADR | UN 3462 6.1/PG 3 | | WGK Germany | 3 | | F | 10-21 | | HazardClass | 6.1 |
| | ALAMETHICIN Usage And Synthesis |
| Uses | Alamethicin is used as a channel-forming ionophore that activates membrane enzymes, membrane channel-forming peptide. It is a 20-amino acid channel-forming peptide antibiotic that can function as a monovalent cation ionophore. Alamethicin has been used to develop methods for the routine assessment of potential new drug candidates to elicit their pharmacokinetic drug interactions. | | Enzyme inhibitor | These voltage-dependent, channel-forming, peptaibol antibiotics
(MWAlamethicin F-30 = 1964.40; CAS 27061-78-5) from the soil fungus
Trichoderma viride NRRL 3199 are membrane-active oligopeptides
isolated from that exhibit anti-bacterial and anti-fungal properties.
Peptaibols are amphipathic, usually highly helical in structure, and
typically form voltage dependent ion channels that uncouple oxidative
phosphorylation, often attended by in bacterial and fungal cell death.
Alamethicin F-30 has the sequence Ac-Aib-Pro-Aib-Ala-Aib-Ala-Gln-Aib-
Val-Aib-Gly-Leu-Aib-Pro-Val-Aib-Aib-Glu-Gln-Phe-OH; Alamethicin F-
50 has the sequence Ac-Aib-Pro-Aib-Ala-Aib-Ala-Gln-Aib-Val-Aib-Gly-
Leu-Aib-Pro-Val-Aib Aib-Gln-Gln-Phe-OH; and Alamethicin II has the
sequence Ac-Aib-Pro-Aib-Ala-Aib-Aib-Gln-Aib-Val-Aib-Gly-Leu-Aib-
Pro-Val-Aib-Aib-Glu-Gln-Phe-OH. Note: The name “peptaibol” is derived
from the prefix “pep-“, the central syllable “aib”, and the suffix “-ol” to
designate its peptide structure, the presence of an a-aminoisobutyryl unit,
and the C-terminal alcohol. |
| | ALAMETHICIN Preparation Products And Raw materials |
|