|
|
| | meproscillarin Basic information |
| Product Name: | meproscillarin | | Synonyms: | meproscillarin;3β-[(4-O-Methyl-6-deoxy-α-L-mannopyranosyl)oxy]-14-hydroxybufa-4,20,22-trienolide;3β-[(6-Deoxy-4-O-methyl-α-L-mannopyranosyl)oxy]-14-hydroxybufa-4,20,22-trienolide;Clift;Meprosillarin;Proscillaridin 4'-methyl ether;Methyl proscillaridin;Methyl proscillaridin A | | CAS: | 33396-37-1 | | MF: | C31H44O8 | | MW: | 544.681 | | EINECS: | 2514936 | | Product Categories: | | | Mol File: | 33396-37-1.mol | 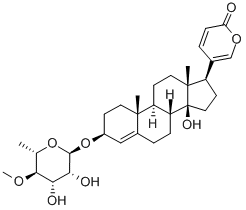 |
| | meproscillarin Chemical Properties |
| Melting point | 213-217℃ | | alpha | D20 -94° (CH3OH) | | Boiling point | 538.46°C (rough estimate) | | density | 1.29 | | refractive index | 1.4900 (estimate) |
| Toxicity | LD50 oral in rat: 79mg/kg |
| | meproscillarin Usage And Synthesis |
| Originator | Clift,Knoll | | Definition | ChEBI: Meproscillarin is a steroid lactone. It is functionally related to a bufanolide. | | Manufacturing Process | 100 g proscillaridin (from Scilla maritima L., enzymatic hydrolysis) was dissolved in 500 ml dry tetrahydrofuran, mixed with 100 ml of triethyl ortoformiate and 50 mg p-tholuene sulfonic acid and stirred for 15 minutes at 20°C.
It was put into a separating funnel and shook with 1 L ethyl acetate and 200 ml of 5% sodium hydroxide. The an organic layer was separated, with 2-3 L water washed (portions 400-500 ml), dried over sodium sulfate and distilled in vacuum to dryness at about 60°C. 121.2 g crude proscillaridin-2,3-ethyl ortoformiate yielded. It was dissolved in 1 L dimethylformamide, mixed with 200 ml methyl iodide and stirred with 20 g 55-60% suspension of sodium hydride at 20°C for 1 hour. 14 L ethyl acetate was added, 5 times with 1-2 L water shook and the organic layer was distilled to 1/4 of volume. The solution of proscillaridin-2,3-ethyl ortho-formiate-4-methyl ester obtained (about 1 L) was mixed with 2 L 0.002 N HCl and stood for 2 hours at 20°C. Then it was neutralized with 0.1 N sodium hydroxide and distilled in vacuum to about 1 L. The solution was shook with 2 L chloroform and 1 L water, organic layer was separated, water layer was 2 times was extracted with still 1 L chloroform and the pooled organic phase dried over sodium sulfate. Then the solvent was removed and 147 g of obtained product was purified by chromatography on silica gel in system chloroform/acetone 4:1.
63 g of crude amorphic 4-O-methylproscillaridin was isolated and recrystallized from methylene chloride/ethyl acetate to give 49.3 g (53% yield) the desired product; MP: 213°-217°C. | | Therapeutic Function | Cardiotonic | | Safety Profile | Poison by ingestion and
intravenous routes. Experimental reproductive effects. When heated to
decomposition it emits acrid smoke and
irritating fumes. |
| | meproscillarin Preparation Products And Raw materials |
|