| | pipradrol Basic information |
| Product Name: | pipradrol | | Synonyms: | Detaril;Pipradol;di(phenyl)-(2-piperidyl)methanol;di(phenyl)-piperidin-2-ylmethanol;di(phenyl)-piperidin-2-yl-methanol;pipradrol;Azacyclononl;XSWHNYGMWWVAIE-UHFFFAOYSA-N | | CAS: | 467-60-7 | | MF: | C18H21NO | | MW: | 267.37 | | EINECS: | 207-394-5 | | Product Categories: | | | Mol File: | 467-60-7.mol | 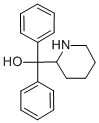 |
| | pipradrol Chemical Properties |
| Melting point | 81-82 °C(Solv: ethanol (64-17-5)) | | Boiling point | 410.55°C (rough estimate) | | density | 1.0449 (rough estimate) | | refractive index | 1.5100 (estimate) | | pka | pKa 9.71 (Uncertain) |
| | pipradrol Usage And Synthesis |
| Originator | Alertonic ,Adcock Ingram Ltd. | | Definition | ChEBI: Pipradrol is a diarylmethane. | | Manufacturing Process | A mixture of 48 g (0.167 mole) of α,α-diphenyl-2-pyridinemethanol hydrochloride (Emraert et al., Ber. 72B, 1188 (1939); 74B, 714 (1940), 160 ml of ethanol, and 3 0.5 g of Adams' platinum catalyst was shaken under an initial hydrogen pressure of 60 pounds. The theoretical amount of hydrogen was absorbed in 5 hours. The reaction mixture was refluxed, diluted with enough water to dissolve all the white solid, and filtered hot from the catalyst. The filtrate was cooled and filtered; yield of 38 g of α,α-diphenyl-alpha-(2piperidyl)methanol white product melting at 308-309°C with decomposition.
A mixture of 3.5 grams (0.013 mole) of the above α,α-diphenyl-alpha-(2piperidyl)methanol, 4 g (0.05 mole) of formaldehyde (37%), and 6 grams (0.1 mole) of formic acid was refluxed for 2 days. The reaction mixture was treated with 1.3 g (0.013 mole) of conc. hydrochloric acid and vacuum distilled on the steam bath. The residue was recrystallized from butanone to give the α,αdiphenyl-alpha-(2-piperidyl)methanol hydrochloride which melted at 228229°C (dec.). | | Brand name | Meratran (Marion Merrell Dow);Alertonic;Gerodryl;Leptidrol;Meratonic;Metadin;Peratran;Piridrol;Stimolag fortis. | | Therapeutic Function | Central stimulant | | World Health Organization (WHO) | Pipradrol, a central nervous system stimulant, was introduced in
1955 for use as an anorexic agent. Pipradrol is controlled under Schedule IV of the
1971 Convention on Psychotropic Substances.
(Reference: (UNCPS4) United Nations Convention on Psychotropic Substances (IV),
, , 1971) |
| | pipradrol Preparation Products And Raw materials |
|