| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
15002134094 |
| Email: |
marketing@targetmol.cn |
| Products Intro: |
Product Name:Indanorex
CAS:16112-96-2
Package:100mg/RMB 17500;25mg/RMB 10600;50mg/RMB 13800
|
| Company Name: |
Leancare Ltd.
|
| Tel: |
+33 962096793 |
| Email: |
enquiry@leancare.co.uk |
| Products Intro: |
|
Indanorex manufacturers
- Indanorex
-

- $2500.00 / 100mg
-
2024-10-28
- CAS:16112-96-2
- Min. Order:
- Purity:
- Supply Ability: 10g
|
| | Indanorex Basic information |
| Product Name: | Indanorex | | Synonyms: | Indanorex;2-(1-Aminopropyl)indan-2-ol;Dietor;JL-11698;2-(1-Aminopropyl)-2,3-dihydro-1H-inden-2-ol;1H-Inden-2-ol, 2-(1-aminopropyl)-2,3-dihydro-;Indanorexum | | CAS: | 16112-96-2 | | MF: | C12H17NO | | MW: | 191.27 | | EINECS: | | | Product Categories: | | | Mol File: | 16112-96-2.mol | 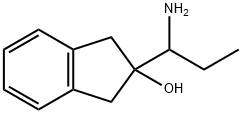 |
| | Indanorex Chemical Properties |
| | Indanorex Usage And Synthesis |
| Originator | Indanorex,Shanghai Lansheng | | Manufacturing Process | 125 g (1.15 mol) trimethylchlorosilane was added to 159 g (1 mol) 2-cyano-
2-hydroxyindane in 600 ml pyridine by stirring for 2 hours, whereupon the
mixture was heated at 1 hour at 40°C for 1 hour. TA light precipitate was
filtered off and washed with 1 liter of benzene. The filtrate was washed with
water, dried over magnesium sulfate and concentrated in vacuum. The residue
was dissolved in benzene 2 times and 2 times the solvent was removed in
vacuum in order to any pyridine and water was present. Yield of silano_x0002_organic compound as a clear brown liquid was 230 g (100%); BP: 95°C/15
mm Hg.
10.5 g magnesium in 30 ml of dry ether was mixed with 55.5 g of ethyl
bromide in 62 ml ether during about 1 hour at the temperature of boiling
ether. After that the mixture was heated to 45°C in order to finish the
synthesis of ethyl magnesium bromide. On cooling to 0°C it was stood for 2
hours and 50 g the above prepared silano-organic compound in 620 ml ether
was added at the temperature about 5°C, whereupon in was stirred else 30
minutes at the ambient temperature and then was placed into ice bath. 82 ml
of methanol was added to the prepared mixture during 1 hour. The
temperature was kept about 18°C. On 30 minutes stirring the mixture was
evaporated to 1/3 volume in vacuum at the temperature about 20°C. The
residue was with ethanol diluted, the ethanol was evaporated, whereupon 100
ml methanol was added. 16.4 g sodium borohydride was added to the
methanol solution for 1 hour at ice cooling, and stirred 2 hours at 3°C. It
stood at ambient temperature overnight. Then the mixture was cooled to
10°C. 250 ml of hydrochloric acid (conc.) was added. After that it was heated
at 45°C for 1 hour. The solvent was removed to dryness in vacuum. The pasty
residue was in 250 ml of water dissolved and with chloroform washed. The 2-
(1-aminopropyl)-2-indanol was precipitated by adding of 750 ml 10% sodium
carbonate to pH 10. It was extracted with chloroform and dried over
magnesium sulfate.
Chloroform was evaporated to give 13 g desired 2-(1-aminopropyl)-2-indanol.
MP: 92°C. Yield 33%. IR spectrum and thin layer chromatography confirmed
the structure of prepared compound. | | Therapeutic Function | Anorexic |
| | Indanorex Preparation Products And Raw materials |
|