| Company Name: |
Chemsky (shanghai) International Co.,Ltd
|
| Tel: |
021-50135380 |
| Email: |
shchemsky@sina.com |
| Products Intro: |
Product Name:Calusterone;(7β,17β)-17-Hydroxy-7,17-diMethylandrost-4-en-3-one; 7β,17-DiMethyltestosterone; 17β-Hydroxy-7β,17-diMethylandrost-4-en-3-one; 7β,17α-DiMethyltestosterone; Methosarb; NSC 88536; U 22550;
CAS:17021-26-0
Purity:98+% Package:1Mg;5Mg;10Mg;50Mg;100Mg;500Mg
|
| Company Name: |
Shanghai Saikerui Biotechnology Co. , Ltd.
|
| Tel: |
021-58000709 15900491054 |
| Email: |
info@scrbio.com |
| Products Intro: |
Product Name:4-ANDROSTEN-7β, 17α-DIMETHYL-17β-OL-3-ONE
CAS:17021-26-0
Purity:98+ Package:1mg;5mg;10mg
|
| Company Name: |
Shanghai Weiyite Biotechnology Co., Ltd
|
| Tel: |
15317051735 |
| Email: |
zhaojing@wytsci.com |
| Products Intro: |
Product Name:Calusterone;Calusterone;7β,17-Dimethyltestosterone;4-Androsten-7β, 17α-Dimethyl-17β-Ol-3-One;
CAS:17021-26-0
Purity:98% Package:1mg
|
|
| | Calusterone Basic information |
| Product Name: | Calusterone | | Synonyms: | (7S,8R,9S,10R,13S,14S,17S)-17-hydroxy-7,10,13,17-tetramethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one;7β,17α-Dimethyl-17β-hydroxyandrost-4-en-3-one;Methosarb;NSC-88536;U-22,550;U-22550;Androst-4-en-3-one, 17-hydroxy-7,17-dimethyl-, (7β,17β)-;4-ANDROSTEN-7β, 17α-DIMETHYL-17β-OL-3-ONE | | CAS: | 17021-26-0 | | MF: | C21H32O2 | | MW: | 316.48 | | EINECS: | | | Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals;Steroids | | Mol File: | 17021-26-0.mol | 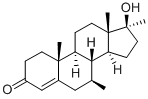 |
| | Calusterone Chemical Properties |
| Melting point | 127-129° | | alpha | D +57° (CHCl3) | | Boiling point | 463.29°C (rough estimate) | | density | 0.9575 (rough estimate) | | refractive index | 1.5100 (estimate) | | solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) | | form | Solid | | pka | 15.13±0.70(Predicted) | | color | White to Off-White |
| | Calusterone Usage And Synthesis |
| Originator | Methosarb,Upjohn,US,1973 | | Uses | Synthetic androgen epimeric with Bolasterone (B674970). Has been used in treatment of breast cancer.
Controlled substance (anabolic steroid). | | Definition | ChEBI: Calusterone is a 3-hydroxy steroid. It has a role as an androgen. | | Manufacturing Process | As described in US Patent 3,029,263, one possibility is a multistep synthesis
starting from 3β,17β-dihydroxy-17α-methyl-5-androstene.
Alternatively, as described in US Patent 3,341,557, 6-dehydro-17-
methyltestosterone may be used as the starting material. A mixture of 0.4 g
of cuprous chloride, 20 ml of 4M methylmagnesium bromide in ether and 60
ml of redistilled tetrahydrofuran was stirred and cooled in an ice bath during
the addition of a mixture of 2.0 g of 6-dehydro-17-methyltestosterone, 60 ml
of redistilled tetrahydrofuran and 0.2 g of cuprous chloride. The ice bath was
removed and stirring was continued for four hours. Ice and water were then
carefully added, the solution acidified with 3 N hydrochloric acid and extracted
several times with ether. The combined ether extracts were washed with a
brine-sodium carbonate solution, brine and then dried over anhydrous
magnesium sulfate, filtered and then poured over a 75-g column of
magnesium silicate (Florisil) packed wet with hexanes (Skellysolve B). The
column was eluted with 250 ml of hexanes, 0.5 liter of 2% acetone, two liters
of 4% acetone and 3.5 liters of 6% acetone in hexanes.
Four 250-ml fractions were collected followed by 150 ml fractions. The
residues from fractions 8 to 16 were combined and rechromatographed over a
125-g column of magnesium silicate. The solumn was eluted with 6% acetone
in hexanes which was collected in 150 ml portions. Fractions 18 to 29 were
combined and dissolved in acetone, decolorized with charcoal, and
recrystallized from acetone. One gram of a crystalline mixture of the 7epimers
of 7,17-dimethyltestosterone was obtained melting at 120° to 140°C. | | Therapeutic Function | Antineoplastic |
| | Calusterone Preparation Products And Raw materials |
|