| Company Name: |
Guangzhou Isun Pharmaceutical Co., Ltd
|
| Tel: |
020-39119399 18927568969 |
| Email: |
isunpharm@qq.com |
| Products Intro: |
Product Name:Violacein from Janthinobacterium lividum
CAS:548-54-9
Purity:>=98% (HPLC) Package:5MG; 25MG; 100MG; 1G
|
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Email: |
orderCN@merckgroup.com |
| Products Intro: |
Product Name:Violacein
CAS:548-54-9
Purity:> 98 % HPLC violacein (minimum 85% violacein) and deoxyviola Package:1mg Remarks:V9389
|
- Violacein
-

- $738.00 / 1mg
-
2024-10-28
- CAS:548-54-9
- Min. Order:
- Purity:
- Supply Ability: 10g
|
| | violacein Basic information |
| Product Name: | violacein | | Synonyms: | violacein;3-[2-(5-Hydroxy-1H-indol-3-yl)-5-oxo-2-pyrrolin-4-ylidene]-1,3-dihydro-2H-indol-2-one;3-[2-(5-Hydroxy-1H-indol-3-yl)-5-oxo-2-pyrrolin-4-ylidene]-2-indolinone;3-[5-(5-Hydroxy-1H-indole-3-yl)-2,3-dihydro-2-oxo-1H-pyrrole-3-ylidene]-1H-indole-2(2H)-one;2-Indolinone, 3-(2-(5-hydroxyindol-3-yl)-5-oxo-2-pyrrolin-4-ylidene)-;3-(2-(5-Hydroxyindol-3-yl)-5-oxo-2-pyrrolin-4-ylidene)-2-indolinone;Brn 0049923;Oxindole, 3-(2-(5-hydroxyindol-3-yl)-5-oxo-2-pyrrolin-4-ylidene)- (6ci) | | CAS: | 548-54-9 | | MF: | C20H13N3O3 | | MW: | 343.34 | | EINECS: | | | Product Categories: | | | Mol File: | 548-54-9.mol | 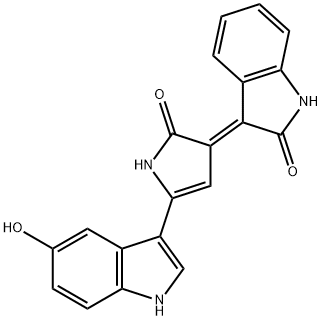 |
| | violacein Chemical Properties |
| Melting point | >350 °C (decomp) | | Boiling point | 821.4±65.0 °C(Predicted) | | density | 1.549±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | H2O: insoluble | | form | A solid | | pka | 9.72±0.40(Predicted) | | color | Brown to black |
| | violacein Usage And Synthesis |
| Description | Violacein is a naturally occurring di-indole-pyrrole violet-blue colored pigment that possesses numerous biological functions such as antimicrobial, antiviral, anticancer, antiulcerogenic, anti-leishmanial, and enzyme modulation properties (Narsing et al. 2017). Violacein is biosynthesized by bacterial species such as Chromobacterium violaceum, Collimonas sp., Pseudoalteromonas sp., Pseudomonas aeruginosa, and Janthinobacterium sp. This natural pigment is used extensively in the cosmetic, food, pharmaceutical, and textile industries (Baiano 2014). | | Uses | Violacein is an intense violet pigment formed by the condensation of two tryptophan units, found in a number of bacteria, notably Chromobacterium violaceum. The regulation of pigment biosynthesis is the chromogenic basis for the use of C. violaceum CV26 for the detection of quorum sensing mediators. Violacein exhibits broad spectrum actvity against bacteria, protozoans (including malaria), viruses and mammalian cell lines. Violacein cell toxicity resembles TNF-α signal transduction. | | Uses | Violacein is tryptpphan-derived pigment produced by environmental bacterial. Violacein inhibits gram-positive pathogens by tearing off cytoplasmic cells as observed in Bacillus subtilis and Staphylococcus aureus cells using fluorescence microscopy. | | Uses | Violacein from Janthinobacterium lividum has been used:
for cell culture assays
as a standard to determine the crude violacein concentration in ethanol extracts of D. violaceinigra str. NI28 cultures
as a standard to identify violacein in the leaf samples of Nicotiana | | Definition | ChEBI: violacein is a member of the class of hydroxyindoles resulting from the formal oxidative coupling between the 3-position of 1,3-dihydro-2H-indol-2-one and the 3-position of 1,3-dihydro-2H-pyrrol-2-one, which is substituted at the 5 position by a 5-hydroxy-1H-indol-3-yl group, where the newly-formed double bond has E configuration. It is a purple chromobacterial pigment that has antibacterial, antifungal, antiprotozoan, and anticancer properties. | | General Description | Violacein from Janthinobacterium lividum is used as a colorant for natural and synthetic fabrics. It functions as a respiratory pigment and regulates tryptophan production. Violacein exhibits anti-protozoal activity. | | Biochem/physiol Actions | Violacein, a violet pigment, is an indole derivative produced by various bacterial strains such as Chromobacterium violaceum, Janthinobacterium lividum, Chromobacterium lividum, and Pseudoalteromonas luteoviolacea. Violacein is a member of a novel class of cytotoxic drugs, which mediate apoptosis. Violacein exhibits antitumoral, antibacterial, antiulcerogenic, antileishmanial, and antiviral activities. Violacein and its β-cyclodextrin complexes trigger apoptosis and differentiation in HL60 leukemic cells. Violacein cytotoxicity is preceded by activation of caspase 8, transcription of NF-κB target genes, and p38-MAPK activation resembling TNF-α signal transduction. |
| | violacein Preparation Products And Raw materials |
|