- Indacaterol Impurity 6
-

- $0.00 / 10mg
-
2025-04-02
- CAS:312753-53-0
- Min. Order: 10mg
- Purity: 95%+
- Supply Ability: 100000
|
| | 5,6-Diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride Basic information |
| Product Name: | 5,6-Diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride | | Synonyms: | 1H-Inden-2-aMine,5,6-diethyl-2,3-dihydro-,hydrochloride (1:1);5,6-Diethyl-2,3-dihydro-1H-inden-2-aMino Hydrochloride;5,6-Diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride 312753-53-0;1H-Inden-2-amine,5,6-diethyl-2,3-dihydro-, hydrochloride;5,6-Diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride;Indacaterol Intermediate 1;5,6-diethylindan-1-one;Indacaterol Impurity 10 | | CAS: | 312753-53-0 | | MF: | C13H20ClN | | MW: | 225.76 | | EINECS: | 691-331-5 | | Product Categories: | Intermediate of Indacaterol | | Mol File: | 312753-53-0.mol | 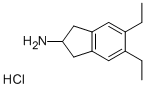 |
| | 5,6-Diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride Chemical Properties |
| Melting point | >250 ºC | | storage temp. | Sealed in dry,Room Temperature | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | color | White | | CAS DataBase Reference | 312753-53-0(CAS DataBase Reference) |
| | 5,6-Diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride Usage And Synthesis |
| Uses | 5,6-Diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride is used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process. | | Uses | 5,6-Diethyl-2,3-dihydro-1H-inden-2-amine Hydrochloride acts as a reagent in the synthetic preparation of quinolinone compounds and it’s formulations in combination with corticosteroids for treatment of airway disorder. | | Synthesis | 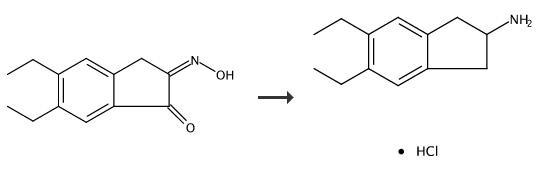
Preparation 4-5,6-Diethyl-indan-2-ylamine Hydrochloride 5,6-Diethyl-3-oxime-1H-indene-1,2(3H)-dione (4.5 g) is added to a mixture of acetic acid (150 mL), and concentrated sulphuric acid (4.5 mL). Pd/C5% (1.5 g) is added, the reaction mixture degassed with nitrogen, and hydrogenated for 5 hours. The catalyst is then removed by filtration, the pH brought to pH 10 with 4M NaOH, and the solution extracted with chloroform. The organic phase is dried with magnesium sulphate, and the solvent removed in vacuo. The residue is redisolved in a minimum amount of ether, and HCl saturated ether added. The white precipitate is filtered and dried to yield the HCl salt of 5,6-diethyl-indan-2-ylamine, a compound of formula XVII where R3, R4 and R7 are H, R5 and R6 are each CH3CH2-, R30 is hydrogen and n is 1. 5,6-Diethyl-indan-2-ylamine Hydrochloride. |
| | 5,6-Diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride Preparation Products And Raw materials |
|