- vanadyl sulfate
-

- $60.00 / 25KG
-
2025-03-21
- CAS:27774-13-6
- Min. Order: 25KG
- Purity: 99.99%
- Supply Ability: 200ton
- Vanadyl Sulfate;VOSO4
-

- $28.00 / 25Kg/Drum
-
2025-03-07
- CAS:27774-13-6
- Min. Order: 25KG
- Purity: 99.5%min
- Supply Ability: 100tons/month
- vanadyl sulfate
-

- $0.00 / 1kg
-
2025-03-07
- CAS:27774-13-6
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 100tons
|
| | Vanadyl sulfate Basic information |
| Product Name: | Vanadyl sulfate | | Synonyms: | ci77940;oxo(sulfato(2-)-o)-vanadiu;oxo(sulfato(2-)-O)-Vanadium;oxo[sulfato(2-)-o]-vanadiu;oxosulfato-vanadiu;oxysulfato-vanadiu;vanadiumoxidesulfate(vo(so4));Vanadium, oxosulfato(2-)-.kappa.O- | | CAS: | 27774-13-6 | | MF: | O5SV | | MW: | 163 | | EINECS: | 248-652-7 | | Product Categories: | metal sulfate;Indoles;Inorganics | | Mol File: | 27774-13-6.mol | 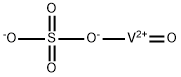 |
| | Vanadyl sulfate Chemical Properties |
| Melting point | 105°C | | density | 2.06[at 20℃] | | solubility | soluble in H2O | | form | Solid | | color | Blue | | Water Solubility | soluble | | Stability: | Stable. Incompatible with strong oxidizing agents. | | InChI | InChI=1S/H2O4S.O.V/c1-5(2,3)4;;/h(H2,1,2,3,4);;/q;;+2/p-2 | | InChIKey | UUUGYDOQQLOJQA-UHFFFAOYSA-L | | SMILES | [O-]([V+2]=O)S([O-])(=O)=O | | LogP | -1.031 (est) | | CAS DataBase Reference | 27774-13-6(CAS DataBase Reference) | | EPA Substance Registry System | Vanadyl sulfate (27774-13-6) |
| | Vanadyl sulfate Usage And Synthesis |
| Chemical Properties | Vanadyl sulfate is a pale blue crystalline powder. It is odorless and very soluble in water. It is denser than water. Contact may irritate skin, eyes, and mucous membranes. It may be toxic if ingested, inhaled, or absorbed through the skin.
 | | Physical properties | The dihydrate, VOSO4.2H2O is a blue black crystalline powder, soluble in water. | | Uses | The dihydrate is a mordant in dyeing and printing fabrics; used in preparing aniline black; a colorant in ceramics to form blue and green glazes; used in making colored glass; and a reducing agent. | | Preparation | Vanadyl sulfate is prepared by passing sulfur dioxide through a cold solution of vanadium pentoxide in sulfuric acid, followed by crystallization:
V2O5 + H2SO4 + H2O + SO2 → 2VOSO4 + 2H2O | | Definition | ChEBI: Vanadyl sulfate is a vanadium coordination entity and a metal sulfate. | | General Description | Vanadyl sulfate is a sulfate of vanadium that occurs naturally as the mineral pauflerite. It is a common source of vanadium in the laboratory. Vanadyl sulfate is an intermediate in the extraction of vanadium from petroleum residues and is also a component of some food supplements and drugs. Vanadium is a transition metal with the chemical symbol V and atomic number 23. The element usually combines with other elements such as oxygen, sodium, sulfur, or chloride, and occurs naturally in about 65 different minerals and in fossil fuel deposits. Vanadium is found in many organisms, and is used by some life forms as an active center of enzymes. (L837, L838, L852) | | Reactivity Profile | Vanadyl sulfate has weak oxidizing or reducing powers. Redox reactions can however still occur. | | Health Hazard | Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | | Fire Hazard | Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways. | | Flammability and Explosibility | Non flammable | | Side effects | Commonly reported side effects of vanadyl sulfate include gastrointestinal issues such as nausea, diarrhea, and stomach cramps. These mild side effects tend to subside with continued use or dose adjustment. However, they can be bothersome and might deter some individuals from using the supplement. More serious risks, though rare, include toxicity from excessive intake. High doses of vanadium compounds can lead to issues such as liver and kidney damage. Therefore, it’s essential to adhere to recommended dosages and avoid excessive use.
| | Safety Profile | A poison and an
inhalation hazard. Poison by intravenous,
intraperitoneal, and subcutaneous routes.
Mutation data reported. When heated to
decomposition it emits toxic fumes of VOx
and SOx. See also SULFATES and
VANADIUM COMPOUNDS. | | Potential Exposure | Vanadyl sulfate is used as a fixative
for textile dyes, a colorant for glass and ceramics; a reducing
agent and a catalyst. | | Shipping | UN2931 Vanadyl sulfate, Hazard Class: 6.1;
Labels: 6.1-Poisonous materials. | | Incompatibilities | Vanadyl sulfate has weak oxidizing or
reducing powers. Redox reactions can however still
occur. Incompatible with oxidizers (chlorates, nitrates,
peroxides, permanganates, perchlorates, chlorine, bromine,
fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides. Vanadyl sulfate may attack metals; sulfates
react with aluminum, magnesium. |
| | Vanadyl sulfate Preparation Products And Raw materials |
|