| | 1-Boc-4-(4-fluoro-phenylamino)-piperidine Basic information |
| Product Name: | 1-Boc-4-(4-fluoro-phenylamino)-piperidine | | Synonyms: | 1-BOC-4-(4-FLUORO-PHENYLAMINO)-PIPERIDINE;1-tert-Butoxycarbonyl-4-[(4-fluorophenyl)amino]piperidine;1,1-dimethylethyl ester CAS 288573-56-8;1-Piperidinecarboxylic acid, 4-[(4-fluorophenyl)amino]-, 1,1-dimethylethyl ester;1-BOC-4-(4-FLUORO-PHENYLAMINO)-PIPERIDINE Basic information;China Tert-Butyl 4- (4-fluoroanilino) Piperidine-1-Carboxylate;KS-0037 powder;4-[(4-Fluorophenyl)Amino]Piperidine-1-Carboxylate | | CAS: | 288573-56-8 | | MF: | C16H23FN2O2 | | MW: | 294.36 | | EINECS: | 200-533-0 | | Product Categories: | 288573-56-8;1;Pharm | | Mol File: | 288573-56-8.mol | 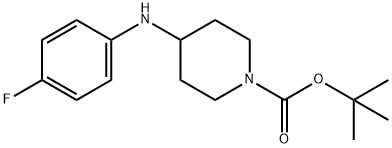 |
| | 1-Boc-4-(4-fluoro-phenylamino)-piperidine Chemical Properties |
| Boiling point | 400.4±40.0 °C(Predicted) | | density | 1.159±0.06 g/cm3(Predicted) | | pka | 5.09±0.20(Predicted) | | InChI | InChI=1S/C16H23FN2O2/c1-16(2,3)21-15(20)19-10-8-14(9-11-19)18-13-6-4-12(17)5-7-13/h4-7,14,18H,8-11H2,1-3H3 | | InChIKey | TUYSUNKDSSHKHM-UHFFFAOYSA-N | | SMILES | N1(C(OC(C)(C)C)=O)CCC(NC2=CC=C(F)C=C2)CC1 |
| | 1-Boc-4-(4-fluoro-phenylamino)-piperidine Usage And Synthesis |
| Chemical Properties | 1-Boc-4-(4-Fluoro-Phenylamino)-Piperidine (Tert-Butyl 4- (4-fluoroanilino) Piperidine-1-Carboxylate) is a white solid.
 | | Uses | 1-Boc-4-(4-fluoro-phenylamino)-piperidine (Tert-Butyl 4- (4-fluoroanilino) Piperidine-1-Carboxylate) is a heterocyclic derivative and can be used as a pharmaceutical intermediate. | | Preparation | Sodium cyanoborohydride (661 mg, 10.0 mmol) was added to a methylene chloride solution (30 ml) of t-butyl 4-oxopiperidine-1-carboxylate (2.66 g, 13.1 mmol), 4-fluoroaniline (1.46 g, 13.1 mmol) and acetic acid (0.750 ml, 13.1 mmol), at 0°C, and stirring was carried out at room temperature for 15 hours. A saturated aqueous sodium hydrogencarbonate solution was added to the reaction solution, followed by extraction with ethyl acetate, and the extract was washed sequentially with water and saline, and dried over anhydrous sodium sulfate. The organic layer was concentrated and the resulting residue was washed with diisopropyl ether, followed by drying to afford 1-Boc-4-(4-Fluoro-Phenylamino)-Piperidine. Yield 80%.
 |
| | 1-Boc-4-(4-fluoro-phenylamino)-piperidine Preparation Products And Raw materials |
|