|
|
| | Quetiapine EP Impurity N Basic information |
| Product Name: | Quetiapine EP Impurity N | | Synonyms: | Quetiapine EP Imp N (base);Quetiapine EP Impurity N/Quetiapine Dipiperazine Diether Impurity;2-(2-(4-(2-(2-(4-(dibenzo[b,f][1,4]thiazepin-11-yl)piperazin-1-yl)ethoxy)ethyl)piperazin-1-yl)ethoxy)ethanol;Quetiapine Impurity 9(Quetiapine EP Impurity N);Ethanol, 2-[2-[4-[2-[2-(4-dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]ethyl]-1-piperazinyl]ethoxy]-;Quetiapine Fumarate EP Impurity N;2-(2-(4-(2-(2-(4-(dibenzo[b,f][1,4]thiazepin-11-yl)piperazin-1-yl)ethoxy)ethyl)piperazin-1-yl)ethoxy)ethanol(Quetiapine EP Impurity N);EP IMPURITY-N | | CAS: | 1800291-86-4 | | MF: | C29H41N5O3S | | MW: | 539.73 | | EINECS: | | | Product Categories: | | | Mol File: | 1800291-86-4.mol | 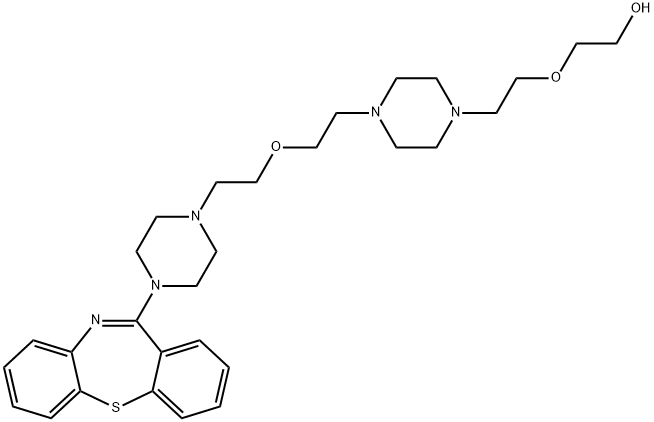 |
| | Quetiapine EP Impurity N Chemical Properties |
| Boiling point | 679.1±65.0 °C(Predicted) | | density | 1.25±0.1 g/cm3(Predicted) | | solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Sparingly) | | pka | 14.41±0.10(Predicted) | | form | Thick Oil to Gel | | color | Light Yellow to Brown | | Stability: | Hygroscopic |
| | Quetiapine EP Impurity N Usage And Synthesis |
| Uses | Quetiapine Impurity-N (Quetiapine EP Impurity N) is a metabolite of Quetiapine, which is approved for the treatment of schizophrenia, bipolar disorder, and along with an antidepressant to treat major depressive disorder. |
| | Quetiapine EP Impurity N Preparation Products And Raw materials |
|