|
|
| | GNE-140 (racemate) Basic information |
| Product Name: | GNE-140 (racemate) | | Synonyms: | GNE-140 (racemate);2,4-Piperidinedione, 3-[(2-chlorophenyl)thio]-6-[4-(4-morpholinyl)phenyl]-6-(3-thienyl)-;3-((2-chlorophenyl)thio)-6-(4-morpholinophenyl)-6-(thiophen-3-yl)piperidine-2,4-dione;GNE-140 racemate
(GNE 140 racemate | | CAS: | 1802977-61-2 | | MF: | C25H23ClN2O3S2 | | MW: | 499.04 | | EINECS: | | | Product Categories: | | | Mol File: | 1802977-61-2.mol | 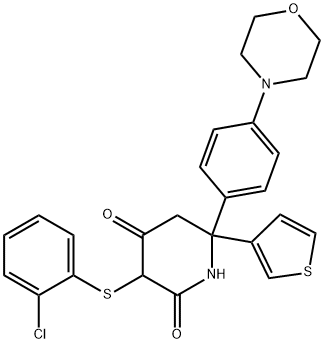 |
| | GNE-140 (racemate) Chemical Properties |
| Boiling point | 739.0±60.0 °C(Predicted) | | density | 1.43±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | DMSO : ≥ 34 mg/mL (68.13 mM) | | pka | 9.61±0.70(Predicted) | | form | Solid | | color | White to off-white |
| | GNE-140 (racemate) Usage And Synthesis |
| Description | GNE-140 racemate is a racemate mixture of (R)-GNE-140 and (S)-GNE-140. GNE-140 racemate is a potent lactate dehydrogenase A (LDHA) inhibitor | | Uses | GNE-140 racemate is a racemate mixture of (R)-GNE-140 and (S)-GNE-140. GNE-140 racemate is a potent lactate dehydrogenase A (LDHA) inhibitor[1][2]. | | in vitro | Increased glucose consumption distinguishes cancer cells from normal cells and is known as the “Warburg effect” because of increased glycolysis. Lactate dehydrogenase A (LDHA) is a key glycolytic enzyme, a hallmark of aggressive cancers, and believed to be the major enzyme responsible for pyruvate-to-lactate conversion. | | References | [1] ?dralevi? M, et al. Double genetic disruption of lactate dehydrogenases A and B is required to ablate the "Warburg effect" restricting tumor growth to oxidative metabolism. J Biol Chem. 2018 Oct 12;293(41):15947-15961. DOI:10.1074/jbc.RA118.004180
[2] Purkey HE, et al. Cell Active Hydroxylactam Inhibitors of Human Lactate Dehydrogenase with Oral Bioavailability in Mice. ACS Med Chem Lett. 2016 Aug 26;7(10):896-901. DOI:10.1021/acsmedchemlett.6b00190 |
| | GNE-140 (racemate) Preparation Products And Raw materials |
|