|
|
| | 2,5-Dimethyl-2,5-hexanediol Basic information |
| Product Name: | 2,5-Dimethyl-2,5-hexanediol | | Synonyms: | 2,5-dimethyl-5-hexanediol;5-Hexanediol,2,5-dimethyl-2;DiMethyl-2,5-hexan;2,5-Dimethyl-2,5-hex;2,5-DiMethyl-2,5-hexanediol, 99% 250GR;2,5-Dimethyl-2,5-hexanediol,99%;2,5-DiMethyl-2,5-hexanediol, 99.5%;2, 5 - diMethyl - 2, 5 - diol | | CAS: | 110-03-2 | | MF: | C8H18O2 | | MW: | 146.23 | | EINECS: | 203-731-5 | | Product Categories: | Organic Building Blocks;Oxygen Compounds;Polyols;Building Blocks;Chemical Synthesis;Organic Building Blocks;Oxygen Compounds;bc0001 | | Mol File: | 110-03-2.mol |  |
| | 2,5-Dimethyl-2,5-hexanediol Chemical Properties |
| Melting point | 86-90 °C (lit.) | | Boiling point | 214-215 °C (lit.) | | density | 0,898 g/cm3 | | vapor pressure | 0.18Pa at 20℃ | | refractive index | 1.4429 (estimate) | | Fp | 126 °C | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | pka | 15.07±0.29(Predicted) | | form | Crystalline Flakes | | color | White | | Odor | mild camphor | | Water Solubility | SOLUBLE | | BRN | 1361437 | | InChIKey | ZWNMRZQYWRLGMM-UHFFFAOYSA-N | | LogP | 0.21 at 23℃ | | CAS DataBase Reference | 110-03-2(CAS DataBase Reference) | | NIST Chemistry Reference | 2,5-Hexanediol, 2,5-dimethyl-(110-03-2) | | EPA Substance Registry System | 2,5-Hexanediol, 2,5-dimethyl- (110-03-2) |
| | 2,5-Dimethyl-2,5-hexanediol Usage And Synthesis |
| Chemical Properties | Colorless or white crystalline flakes. M.P. 93°C.
B.P. 213°C. Soluble in water (20°C 140g/L), miscible with alcohol and
most oils. Faint, camphorlike odor. | | Uses | 2,5-Dimethyl-2,5-hexanediol is used as an intermediate in the preparation of 2.5-Dimethyl-2.5-bis(tertbutyl-peroxy)hexane to produce polyethylene copolymers and polyethylene rubbers. It is also could be used to prepared nickel-containing Complex Reducing Agents (termed NiCRA)[1]. | | Uses | 2,5-Dimethyl-2,5-hexanediol was used in the synthesis of six- and seven-membered heterocyclic boron compounds containing intramolecular N-B bond. | | Preparation | The raw materials 2,5-Dimethyl-3-hexyne-2,5-diol (37.4 g, 263 mmol), ethyl acetate (60 mL), and Pd/C catalyst (0.285 g, 2.63 mmol, 1 mol %) were added to an autoclave. The hydrogen gas was slowly added to 10 bar. When the hydrogenation reaction was performed for 12 hours and then removed the hydrogen gas. Finally, 2,5-Dimethyl-2,5-hexanediol is obtained by purification.
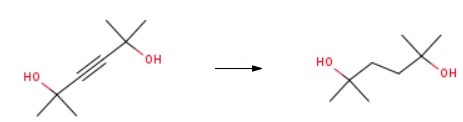 | | Synthesis Reference(s) | Synthesis, p. 165, 1981 DOI: 10.1055/s-1981-29377 | | General Description | 2,5-Dimethyl-2,5-hexanediol on heteropoly acid catalyzed dehydration yields cyclic ethers via stereospecific intramolecular SN2 mechanism. It reacts with nitriles in concentrated sulfuric acid to yield Δ1-pyrrolines. | | Purification Methods | Purify the diol by fractional crystallisation. Then the diol is dissolved in hot acetone, treated with activated charcoal, and filtered while hot. The solution is cooled and the diol is filtered off and washed well with cold acetone. The crystallisation process ia repeated several times, and the crystals are dried under a vacuum in a freeze-drying apparatus [Goates et al. J Chem Soc, Faraday Trans 1 78 3045 1982]. [Beilstein 1 IV 2600.] | | References | [1] Feghouli G, et al. Activation of reducing agents. Sodium hydride containing complex reducing agents 29. Epimerization of alcohols by nickel-containing complex reducing agents (NiCRA). Tetrahedron Letters, 1988; 9: 285–290. |
| | 2,5-Dimethyl-2,5-hexanediol Preparation Products And Raw materials |
| Raw materials | Nickel-->3-Methyl butynol-->3-HEXYNE-->2,5-Hexanediol-->2,5-DIMETHYL-2-HEXANOL-->2,5-Dimethyl-3-hexyne-2,5-diol-->2,5-DIMETHYLHEXANE | | Preparation Products | Bexarotene-->1,1,4,4-Tetramethyl-1,2,3,4-tetrahydronaphthalene-->2,5-DICHLORO-2,5-DIMETHYLHEXANE-->1,1,4,4,6-Pentamethyl-1,2,3,4-tetrahydronaphthalene-->Naphthalene,6-chloro-1,2,3,4-tetrahydro-1,1,4,4-tetramethyl- |
|