|
|
| | Titanium tetraisopropanolate Basic information | | Reactions |
| | Titanium tetraisopropanolate Chemical Properties |
| Melting point | 14-17 °C(lit.) | | Boiling point | 232 °C(lit.) | | density | 0.96 g/mL at 20 °C(lit.) | | vapor pressure | 60.2hPa at 25℃ | | refractive index | n20/D 1.464(lit.) | | Fp | 72 °F | | storage temp. | Flammables area | | solubility | Soluble in anhydrous ethanol, ether, benzene and chloroform. | | form | Liquid | | Specific Gravity | 0.955 | | color | Colorless to pale yellow | | Water Solubility | HYDROLYSIS | | FreezingPoint | 14.8℃ | | Sensitive | Moisture Sensitive | | Hydrolytic Sensitivity | 7: reacts slowly with moisture/water | | Merck | 14,9480 | | BRN | 3679474 | | Stability: | Stable, but decomposes in the presence of moisture. Incompatible with aqueous solutions, strong acids, strong oxidizing agents. Flammable. | | InChIKey | VXUYXOFXAQZZMF-UHFFFAOYSA-N | | LogP | 0.05 | | CAS DataBase Reference | 546-68-9(CAS DataBase Reference) | | EPA Substance Registry System | 2-Propanol, titanium(4+) salt (546-68-9) |
| | Titanium tetraisopropanolate Usage And Synthesis |
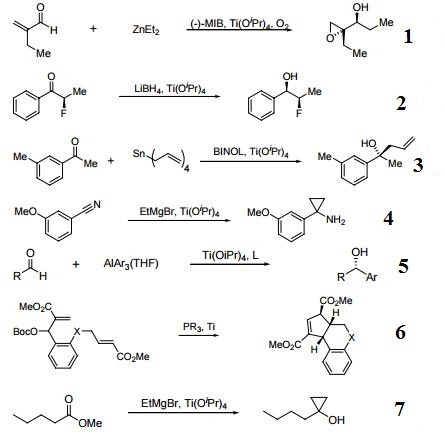
| Reactions |
- Catalyst for the synthesis of acyclic epoxy alcohols and allylic epoxy alcohols.
- Useful for diastereoselective reduction of alpha-fluoroketones.
- Catalyzes the asymmetric allylation of ketones.
- Reagent for the synthesis of cyclopropylamines from aryl and alkenyl nitriles.
- Useful for racemic and/or enantioselective addition of nucleophiles to aldehydes, ketones and imines.
- Catalytic intramolecular formal [3+2] cycloaddition.
- Catalyst for the synthesis of cyclopropanols from esters and organomagnesium reagents
| | Chemical Properties | colourless to light yellow liquid | | Uses | Titanium(IV) isopropoxide is used as a precursor for the preparation of titanium and barium-strontium-titanate thin films. It is useful to make porous titanosilicates and potential ion-exchange materials for cleanup of radioactive wastes. It is an active component of Sharpless epoxidation as well as involved in the synthesis of chiral epoxides. In Kulinkovich reaction, it is involved as a catalyst in the preparation of cyclopropanes. | | Uses | Catalyst especially for asymmetric induction in organic syntheses; in preparation of nanosized TiO2. Complexing agent in sol-gel process. | | Definition | ChEBI: Titanium(IV) isopropoxide is a titanium coordination entity consisting of a titanium(IV) cation with four propan-2-olate anions as counterions. | | General Description | Tetraisopropyl titanate appears as a water-white to pale-yellow liquid with an odor like isopropyl alcohol. About the same density as water. Vapors heavier than air. | | Air & Water Reactions | Highly flammable. Fumes in air. Soluble in water. Decomposes rapidly in water to form flammable isopropyl alcohol. | | Reactivity Profile | Metal alkyls, such as TETRAISOPROPYL TITANATE, are reducing agents and react rapidly and dangerously with oxygen and with other oxidizing agents, even weak ones. Thus, they are likely to ignite on contact with alcohols. | | Health Hazard | Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. | | Flammability and Explosibility | Flammable | | reaction suitability | core: titanium
reagent type: Lewis acid
reagent type: catalyst | | Purification Methods | Dissolve it in dry *C6H6 , filter if a solid separates, evaporate and fractionate. It is hydrolysed by H2O to give solid Ti2O(iso-OPr)2 m ca 48o. [Bradley et al. J Chem Soc 2027, 1952, Bradley et al. J Chem Soc 469 1957, Beilstein 1 II 328, 1 IV 1469S.] |
| | Titanium tetraisopropanolate Preparation Products And Raw materials |
|