- 2-Methoxynaphthalene
-

- $0.00 / 25Kg/Drum
-
2025-04-16
- CAS:93-04-9
- Min. Order: 1KG
- Purity: ≥99%
- Supply Ability: 2000mt/year
- 2-Methoxynaphthalene
-

- $45.00 / 1kg
-
2025-04-16
- CAS:93-04-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 tons
- 2-Methoxynaphthalene
-

- $30.00 / 1KG
-
2025-04-15
- CAS:93-04-9
- Min. Order: 50KG
- Purity: 99%
- Supply Ability: 500000kg
|
| | 2-Methoxynaphthalene Basic information |
| | 2-Methoxynaphthalene Chemical Properties |
| Melting point | 70-73 °C (lit.) | | Boiling point | 274 °C (lit.) | | density | 1.064 g/mL at 25 °C (lit.) | | vapor pressure | 1.097Pa at 25℃ | | refractive index | 1.5440 (estimate) | | FEMA | 4704 | BETA-NAPHTHYL METHYL ETHER | | Fp | 272-274°C | | storage temp. | Sealed in dry,Room Temperature | | solubility | H2O: soluble (completely) | | form | Crystalline Solid | | pka | 0[at 20 ℃] | | color | White to yellow-brown | | Odor | at 1.00 % in dipropylene glycol. sweet naphthyl floral orange blossom acacia neroli | | Odor Type | naphthyl | | biological source | synthetic | | Water Solubility | INSOLUBLE | | Merck | 14,5997 | | JECFA Number | 1257 | | BRN | 1859408 | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. | | InChIKey | LUZDYPLAQQGJEA-UHFFFAOYSA-N | | LogP | 3.318 at 25℃ | | CAS DataBase Reference | 93-04-9(CAS DataBase Reference) | | NIST Chemistry Reference | Naphthalene, 2-methoxy-(93-04-9) | | EPA Substance Registry System | Naphthalene, 2-methoxy- (93-04-9) |
| Safety Statements | 22-24/25 | | RIDADR | UN 3077 9 / PGIII | | WGK Germany | 2 | | RTECS | QJ9468750 | | TSCA | Yes | | HS Code | 29093090 | | Toxicity | LD50 oral in rat: > 5gm/kg |
| | 2-Methoxynaphthalene Usage And Synthesis |
| Description | β-Naphthyl methyl ether has an intensely sweet, floral odor suggestive of orange blossoms. It is free from naphthol by-odor. It has a sweet, strawberry taste. This may be prepared from potassium β-naphthol and methyl chloride at 300°C; by methylation of β-naphthol with dimethyl sulfate or by direct esterification with methyl alcohol. | | Chemical Properties | β-Naphthyl methyl ether has an intensely sweet, floral odor suggestive of orange blossoms; free from naphthol by-odor.
It has a sweet, strawberry taste | | Chemical Properties | Methyl 2-Naphthyl Ether forms white crystals (mp
73–74°C) with an intense orange blossom odor. | | Chemical Properties | white powder | | Uses | 2-methoxynaphthalene acylation was used as a model reaction to study the catalytic benefits of delamination. It was also used to study the alkali-metal-mediated manganation (AMMMn) reactions. | | Uses | 2-methoxynaphthalene acylation is used as a model reaction to study the catalytic benefits of delamination. It was also used to study the alkali-metal-mediated manganation (AMMMn) reactions. | | Uses | 2-Methoxynaphthalene is an Impurity of the non-steroidal anti-inflammatory Naproxen (N377525). | | Preparation | From postassium β-naphthol and methyl chloride at 300°C; by methylation of β-naphthlol with dimethyl sulfate or by
direct esterification with methyl alcohol | | Definition | ChEBI: 2-Methoxynaphthalene is a member of naphthalenes. | | Synthesis Reference(s) | Tetrahedron, 48, p. 6439, 1992 DOI: 10.1016/S0040-4020(01)88233-8
Tetrahedron Letters, 22, p. 3463, 1981 DOI: 10.1016/S0040-4039(01)81932-8 | | Flammability and Explosibility | Not classified | | Synthesis | Preparation of 2-Methoxynaphthalene from 2-naphthol.
Principle: Phenols can be methylated to give methyl ethers. Methylation can be done either by using diazomethane or dimethyl sulphate in alkaline medium.
Reaction:
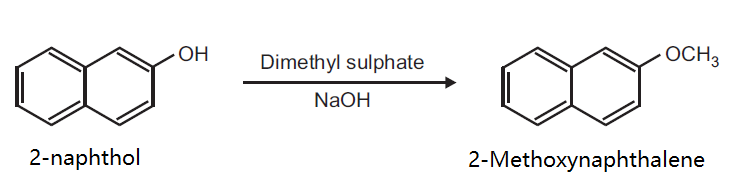
Procedure: Take 0.5 g 2-naphthol and 0.2 g NaOH in 5 ml distilled water in a beaker (25 ml). Heat on a wire gauze to obtain a clear solution. Cool the solution (10-15°C) and then add 0.35 ml dimethyl sulphate drop wise. After the addition is over, warm the mixture for one hour at 70-80°C and then cool. Filter the product and wash it with 10% sodium hydroxide solution and then with water. Dry the product, record the practical yield and re-crystallize it.
Re-crystallization: Dissolve the crude product in minimum amount of ethyl alcohol in a beaker by heating on a water bath. Filter the hot solution and cool the filtrate. Filter the white crystals of the product. Dry and record the melting point and TLC (using toluene as solvent). | | Purification Methods | Fractionally distil the ether under vacuum. Crystallise it from absolute EtOH, aqueous EtOH, *C6H6, pet ether or n-heptane, and dry it under vacuum in an Abderhalden pistol or distil it in vacuo. The picrate has m 118o (from EtOH or CHCl3). [Kikuchi et al. J Phys Chem 91 574 1987, Beilstein 6 III 2969, 6 IV 4257.] |
| | 2-Methoxynaphthalene Preparation Products And Raw materials |
|