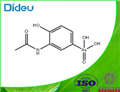
ACETARSONE manufacturers
- ACETARSONE USP/EP/BP
-
- $1.10 / 1g
-
2021-06-18
- CAS:97-44-9
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
|
| | ACETARSONE Basic information |
| Product Name: | ACETARSONE | | Synonyms: | paaroxyl;stovarsole;acetphenarine;amasan;ethrlich594;Acetarsone;Devegan;Qrarsan;Qsarsal;Osarsol;Osvarsan;Paroxyl;Sanogyl;Spirocid;S.V.C;Monargan;Kharophen;Qrarsan;Qsarsal | | CAS: | 97-44-9 | | MF: | C8H10AsNO5 | | MW: | 275.09 | | EINECS: | 202-582-3 | | Product Categories: | STOVARSOL | | Mol File: | 97-44-9.mol | 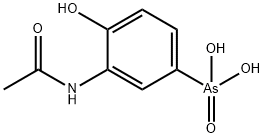 |
| | ACETARSONE Chemical Properties |
| Melting point | 220.5°C | | Boiling point | 72.17°C | | density | 1.0325 g/cm3(Temp: 15 °C) | | storage temp. | Store at RT | | solubility | DMSO (Slightly), Methanol (Slightly, Heated, Sonicated) | | form | solid | | pka | pKa 3.73 (H2O t=26) (Uncertain);7.9 (Uncertain); 9.3(H2O) (Uncertain) | | color | Crystalline material | | Merck | 13,53 |
| Hazard Codes | T,N | | Risk Statements | 23/25-50/53 | | Safety Statements | 20/21-28-45-60-61 | | RIDADR | UN 3465 6.1/PG 2 | | WGK Germany | 3 | | RTECS | CF8400000 | | HazardClass | 6.1(b) | | PackingGroup | III | | Toxicity | MLD in rabbits, cats (mg/kg): 125-150, 150-175 orally (Anderson, Leake) |
| | ACETARSONE Usage And Synthesis |
| Originator | Acetarsol ,Cyklo Pharma
Chem Pvt. Ltd. | | Uses | antiprotozoal; diethylamine salt as antiphilitic | | Definition | ChEBI: Acetarsol is a member of acetamides and an anilide. | | Manufacturing Process | 1 part of 4-chloroaniline is dissolved with 2 parts of concentrated hydrochloric
acid (specific gravity 1.16) and 10 parts of water, and diazotised in the usual
manner. 3 parts of sodium arsenite are introduced into the diazo solution thus
obtained, the sodium arsenite being dissolved in 5 parts of water and 1 part
of 96% ethanol. The solution is heated slowly to 70°C. When evolution of
nitrogene ceased, it is filtered from separated oil and the addition of
hydrochloric acid precipitates the 4-chlorophenylarsonic acid, which
crystallizes out in the form of white needles.
By action HNO3/H2SO4 on 4-chlorophenylarsonic acid is obtained 4-chloro-3-
nitrophenylarsonic acid which is converted at 100°C with 33% aqueous
solution of sodium hydroxide to 4-hydroxy-3-nitrophenylarsonic acid. After
reduction of NO2 group of 4-hydroxy-3-nitrophenylarsonic acid by the action
of Na2S2O3 or Fe/NaOH is obtained 3-amino-4-hydroxyphenylarsonic acid.
From 3-amino-4-hydroxyphenylarsonic acid and acetic anhydride is prepared
N-acetyl-4-hydroxy-m-arsanilic acid. | | Brand name | 190 f;Acetarsolum;Amarson;Arsabott;Auryphan;Chrlich 594;Collarsin;Edoiacolo;Ehrlich 594;F 190;Fluryl;Fourneau 190;Kharophene;Neo-vagex;Pallacid;Trichovan;Vagipurin;Vagisep;Vagival. | | Therapeutic Function | Antiprotozoal, Tonic | | World Health Organization (WHO) | Acetarsol, which has antiprotozoal and antitrichomonal activity,
has largely been discarded for systemic use because of its potential to cause
systemic poisoning. However, topical preparations for vaginal trichomoniasis are
still available and it is included in low concentrations (equal to or less than 0.45%)
in some medicated toothpastes. | | Safety Profile | Poison by ingestion and intravenous routes. Human systemic effects by ingestion: respiratory system, endocrine system, dermatitis, and fever. Human systemic effects by intravagmal route: hallucinations, dlstorted perceptions, convulsions, nausea or vomiting, decreased urine volume, and fever. Mutation data reported. See also ARSENIC COMPOUNDS. When heated to decomposition it emits very toxic fumes of NOx, and As | | Purification Methods | It crystallises from water in colourless prisms. It decomposes slowly on prolonged boiling in H2O or dilute alkalis. The N-propionyl derivative recrystallises from H2O with m 228-229o(dec). [Raiziss & Fisher J Am Chem Soc 48 1323 1926, Hewitt & King J Chem Soc 823 1926, Beilstein 16 I 491, 16 II 521, 16 III 1129.] |
| | ACETARSONE Preparation Products And Raw materials |
|