- Afloqualone USP/EP/BP
-
- $1.10 / 1g
-
2021-07-08
- CAS:56287-74-2
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
- Afloqualone
-

- $1.00 / 1kg
-
2019-07-06
- CAS: 56287-74-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100KG
|
| | Afloqualone Basic information |
| Product Name: | Afloqualone | | Synonyms: | Afloqualone 6-Amino-2-(fluoromethyl)-3-(2-methylphenyl)-quinazolin-4-one;6-Amino-2-fluoromethyl-3-(o-tolyl)-quinazolin-4(3H)-one;H-495;HQ 495;HQ-495;Afloquanone;6-aMino-2-fluoroMethyl-3-(o-tolyl)-4(3h)-quinaz...;6-amino-2-(fluoromethyl)-3-(2-methylphenyl)-4(3h)-quinazolinon | | CAS: | 56287-74-2 | | MF: | C16H14FN3O | | MW: | 283.3 | | EINECS: | 1592732-453-0 | | Product Categories: | API;Other APIs | | Mol File: | 56287-74-2.mol | 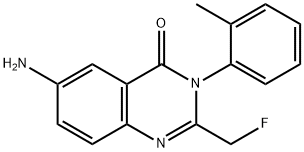 |
| | Afloqualone Chemical Properties |
| Melting point | 195-196°C | | Boiling point | 492.5±55.0 °C(Predicted) | | density | 1.2529 (estimate) | | storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | pka | 2.73±0.70(Predicted) | | color | White to Off-White | | InChI | InChI=1S/C16H14FN3O/c1-10-4-2-3-5-14(10)20-15(9-17)19-13-7-6-11(18)8-12(13)16(20)21/h2-8H,9,18H2,1H3 | | InChIKey | VDOSWXIDETXFET-UHFFFAOYSA-N | | SMILES | N1C2=C(C=C(N)C=C2)C(=O)N(C2=CC=CC=C2C)C=1CF | | CAS DataBase Reference | 56287-74-2(CAS DataBase Reference) |
| Toxicity | LD50 in mice (mg/kg): 315.1 i.p. (Tani) |
| | Afloqualone Usage And Synthesis |
| Description | Afloqualone is a centrally acting muscle relaxant useful in the management of
various spastic conditions, including cerebral palsy, cervical spondylosis, and
multiple sclerosis. It is closely related to the hypnotidsedative methaqualone. | | Description | Afloqualone (Item No. 21834) is an analytical reference standard categorized as a quinazolinone. This product is intended for research and forensic applications. | | Originator | Tanabe (Japan) | | Uses | Afloqualone acts as a muscle relaxant in humans and may be used in the treatment of muscle spasms affecting rheumatoid arthritis. | | Definition | ChEBI: Afloqualone is an organic molecular entity. | | Manufacturing Process | 14.4 g (0.053 mol) of N-(2-amino-5-nitrobenzoyl)-o-toluidine and 6.3 g (0.08
mol) of pyridine are dissolved in 300 ml of tetrahydrofuran. 12.2 g (0.126
mol) of fluoroacetyl chloride are added to the solution for 10 minutes under
ice-cooling. The solution is stirred at the same temperature for 30 minutes
and then at room temperature for 2.5 hours. The reaction solution is allowed
to stand at room temperature overnight. The crystalline precipitate is collected
by filtration, washed with water and then dried. 16.4 g of N-(2-
fluoroacetamido-5-nitrobenzoyl)-o-toluidine are obtained. Yield: 93.7%; MP
238-239°C.
16.5 g (0.05 mol) of N-(2-fluoroacetamido-5-nitrobenzoyl)-o-toluidine and
25.5 g (0.25 mol) of acetic acid anhydride are dissolved in 250 ml of glacial
acetic acid. The solution is refluxed for 2 hours under heating. Then, the
reaction solution is evaporated to remove solvent. The residue thus obtained
is poured into ice-water, and the aqueous mixture is adjusted to pH 9 with
potassium carbonate. The crystalline precipitate is collected by filtration. 15.5
g of 2-fluoromethyl-3-(o-tolyl)-6-nitro-4(3H)-quinazolinone are obtained.
Yield: 98.7%; MP 155-158°C (recrystallized from ethanol).
A mixture of 2.0 g (0.064 mol) of 2-fluoromethyl-3-(o-tolyl)-6-nitro-4(3H)-
quinazolinone, 0.2 g of 5% palladium-carbon and 100 ml of acetic acid is
shaken for 30 minutes in hydrogen gas. The initial pressure of hydrogen gas is
adjusted to 46 lb and the mixture is heated with an infrared lamp during the
reaction. After 30 minutes of this reaction, the pressure of hydrogen gas
decreases to 6 lb. After the mixture is cooled, the mixture is filtered to
remove the catalyst. The filtrate is evaporated to remove acetic acid, and the
residue is dissolved in chloroform. The chloroform solution is washed with 5%
aqueous sodium hydroxide and water, successively. Then, the solution is dried
and evaporated to remove solvent. The oily residue thus obtained is dissolved
in 2 ml of chloroform, and the chloroform solution is passed through a column
of 200 g of silica gel. The silica gel column is eluted with ethyl acetatebenzene
(1:1). Then, the eluate is evaporated to remove solvent. The crude
crystal obtained is washed with isopropyl ether and recrystallized from isopropanol. 0.95 g of 2-fluoromethyl-3-(o-tolyl)-6-amino-4(3H)-quinazolinone
is obtained. Yield: 52.5%; MP 195-196°C. | | Brand name | AROFUTO | | Therapeutic Function | Muscle relaxant |
| | Afloqualone Preparation Products And Raw materials |
|