- Pipemidic acid
-

- $0.00 / 1kg
-
2025-04-14
- CAS:51940-44-4
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1000
- Pipemidic acid
-

- $40.00 / 1kg
-
2025-03-07
- CAS:51940-44-4
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons
- Pipemidic acid
-

- $55.00 / 500mg
-
2024-11-19
- CAS:51940-44-4
- Min. Order:
- Purity: 99.9%
- Supply Ability: 10g
|
| | Pipemidic acid Basic information |
| Product Name: | Pipemidic acid | | Synonyms: | 8-ETHYL-5,8-DIHYDRO-5-OXO-2-[1-PIPERAZINYL]PYRIDO[2,3-D]-PYRIMIDINE-6-CARBOXYLIC ACID;LABOTEST-BB LT00772244;PIPEMIDIC ACID;1489rb;pipemid;piperamicacid;pipram;pyrido(2,3-d)pyrimidine-6-carboxylicacid,5,8-dihydro-8-ethyl-5-oxo-2-(1-piper | | CAS: | 51940-44-4 | | MF: | C14H17N5O3 | | MW: | 303.32 | | EINECS: | 257-530-2 | | Product Categories: | PIPEDAC | | Mol File: | 51940-44-4.mol | 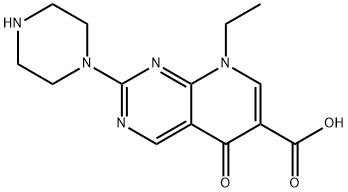 |
| | Pipemidic acid Chemical Properties |
| Melting point | 251-255℃ | | Boiling point | 717℃ | | density | 1.1931 (rough estimate) | | refractive index | 1.7000 (estimate) | | Fp | >110°(230°F) | | storage temp. | Amber Vial, -20°C Freezer, Under inert atmosphere | | solubility | 1 M NaOH: soluble50mg/mL | | pka | 4.14±0.20(Predicted) | | form | Powder | | color | White to off-white | | Water Solubility | Soluble in 1N sodium hydroxide or in DMSO. Insoluble in water | | Sensitive | Light Sensitive | | CAS DataBase Reference | 51940-44-4(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 42/43 | | Safety Statements | 22-36/37-45 | | WGK Germany | 2 | | RTECS | UV1153800 | | HS Code | 29335990 | | Toxicity | LD50 in mice (mg/kg): 4000 orally; 1000 i.p., 50 i.v. (Ficicchia) |
| | Pipemidic acid Usage And Synthesis |
| Chemical Properties | The substance is a light yellow crystalline powder with an odorless, bitter taste. It has a melting point range of 251-255°C, with decomposition. It is soluble in glacial acetic acid, but only slightly soluble in methanol, acetone, and DMF. It is extremely difficult to dissolve in water, ethanol, and chloroform, and is insoluble in benzene. However, it can be dissolved in dilute alkali or acid solutions. | | Originator | Pipram ,Bellon, France ,1975 | | Uses | Pipemidic acid is a quinolone antibacterial drug that is primarily used to treat gram-negative infections of the urinary tract. It is also an antibiotic and cell selection agent. Unlike nalidixic acid, pipemidic acid has a broader spectrum of antibacterial activity and is effective for treating urinary tract and ENT infections. | | Preparation | The synthesis of pipemidic acid involves a series of steps. First, ethyl 2,4-dichloropyrimidine-5-carboxylate is condensed and cyclized with ethyl β-ethylaminopropionate to yield 2-chloro-5-hydroxy-7,8-dihydro-8-ethylpyridine[2,3-d]pyrimidine-6-carboxylate. This compound is then brominated and subsequently condensed with piperazine. The resulting product is hydrolyzed and neutralized to obtain pipemidic acid. | | Definition | ChEBI: Pipemidic acid is a pyridopyrimidine that is 5-oxo-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylic acid substituted at position 2 by a piperazin-1-yl group and at position 8 by an ethyl group. A synthetic broad-spectrum antibacterial, it is used for treatment of gastrointestinal, biliary, and urinary infections. It has a role as an antibacterial drug and a DNA synthesis inhibitor. It is a monocarboxylic acid, an amino acid, a N-arylpiperazine, a pyridopyrimidine and a quinolone antibiotic. | | Manufacturing Process | A mixture containing 1.33 g of 5,8-dihydro-8-ethyl-2-methylthio-5oxopyridol[2,3-d]pyrimidine-6-carboxylic acid, 1.94 g of piperazine hexahydrate and 20 ml of dimethyl sulfoxide was heated at 110°C for 1 hour with stirring. The separated solid was collected by filtration, washed with ethanol, and then dried at such a temperature that did not rise above 50°C to give 1.57 g of the trihydrate of the product as nearly colorless needles, MP 253° to 255°C.
The starting material may be produced by reacting 6-amino-2methylthiopyrimidine with ethoxymethylene malonic acid diethyl ester. The intermediate thus produced is converted by boiling in diphenyl ether to 6ethoxycarbonyl-2-methylthio-5-oxo-5,8-dihydropyrido[2,3-d]pyrimidine. That is hydrolyzed by sodium hydroxide to cleave the ethoxy group and then ethylated with diethyl sulfate to give the starting material. | | Therapeutic Function | Antibacterial (urinary) | | General Description | Chemical structure: quinolone | | Pharmaceutical Applications | An orally administered pyridopyrimidine derivative with a 7-piperazinyl moiety. The piperazinyl moiety at C-7 increases in-vitro activity against Ps. aeruginosa. Pipemidic acid is inactive against Gram-positive bacteria or anaerobes
It is well absorbed orally. The drug is rapidly metabolized, primarily to acetyl, formyl and oxo derivatives, which exhibit much reduced antibacterial activity. It is eliminated in the urine, 50–85% of a dose appearing over the first 24 h, less than 2% as inactive metabolites. Non-renal clearance accounts for 10–40% of a dose in the young, rising to 40–70% in elderly subjects, thereby compensating for possible renal insufficiency. No dosage adjustment is necessary in patients with mild renal insufficiency. Some of the drug is eliminated in the bile and a significant portion appears in the feces.
Nausea and vomiting are common; dizziness, weakness and grand mal seizures have been observed, principally in the elderly. A number of reactions have been sufficiently severe to require discontinuation of therapy. Clinical use is restricted to urinary tract infections. | | Biochem/physiol Actions | Pipemidic acid modulates (inhibits) bacterial DNA gyrase-dependent processes such as DNA polymerization, (ATP-dependent) DNA supercoiling, and chromosome fragmentation. Pipemidic acid has been shown to induce inhibition of lymphocyte DNA synthesis. | | References | Comprehensive Heterocyclic Chemistry II Volume 7, 1996, Pages 561-624 DOI: 10.1016/B978-008096518-5.00160-X
A New Approach for Improving the Antibacterial and Tumor Cytotoxic Activities of Pipemidic Acid by Including It in Trimethyl-β-cyclodextrin doi:10.3390/ijms20020416
https://pubchem.ncbi.nlm.nih.gov/compound/Pipemidic-acid
Pipemidic Acid, a New Antibacterial Agent Active Against Pseudomonas aeruginosa: In Vitro Properties DOI: 10.1128/aac.8.2.132 |
| | Pipemidic acid Preparation Products And Raw materials |
|