- Iloprost
-

- $590.00 / 1mg
-
2024-10-28
- CAS:78919-13-8
- Min. Order:
- Purity:
- Supply Ability: 10g
- ILOPROST
-

- $7.00 / 1KG
-
2019-07-10
- CAS: 78919-13-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100KG
|
| | ILOPROST Basic information |
| Product Name: | ILOPROST | | Synonyms: | (16r,s)-methyl-18,18,19,19-tetradehydro-6a-carbaprostaglandini(sub2);1h)-pentalenylidene)-;pentanoicacid,5-(hexahydro-5-hydroxy-4-(3-hydroxy-4-methyl-1-octen-6-ynyl)-2(;zk36374;6,9ALPHA-METHYLENE-11ALPHA,15S-DIHYDROXY-16-METHYL-PROSTA-5E,13E-DIEN-18-YN-1-OIC ACID;Endoprost;Ilomedin;(5E)-5-[(3aS,4R,5R,6aS)-Hexahydro-5-hydroxy-4-[(1E,3S)-3-hydroxy-4-methyl-1-octen-6-ynyl]-2(1H)-pentalenylidene]pentanoicacid | | CAS: | 78919-13-8 | | MF: | C22H32O4 | | MW: | 360.49 | | EINECS: | | | Product Categories: | Chiral Reagents;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;APIs | | Mol File: | 78919-13-8.mol | 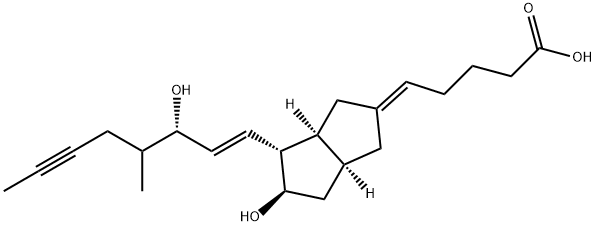 |
| | ILOPROST Chemical Properties |
| Boiling point | 539.2±50.0 °C(Predicted) | | density | 1.210±0.06 g/cm3(Predicted) | | storage temp. | -20°C | | solubility | Soluble in methyl acetate | | pka | 4.77±0.10(Predicted) | | form | film | | color | colorless | | Stability: | Light Sensitive |
| | ILOPROST Usage And Synthesis |
| Description | Iloprost is a stable epoprostenol derivative which acts as an inhibitor of platelet
aggregation and is recommended for the treatment of peripheral vascular diseases. The
compound was shown to be a potent agonist at PGE2-sensitive and
prostacyclin-sensitive receptors in animal studies. Iloprost is administered by IV
infusion and has been reported to have beneficial effects in Buerger's and Raynaud's
diseases as well as peripheral artery occlusive disease. | | Chemical Properties | Colourless Oil | | Chemical Properties | Quinone exists as a large, yellow, monoclinic prism with an irritating odor. Quinone is
extensively used in the dye, textile, chemical, tanning, and cosmetic industries. In chemical
synthesis for hydroquinone and other chemicals, quinone is used as an intermediate.
It is also used in the manufacturing industries and chemical laboratories associated with
protein fi ber, photographic fi lm, hydrogen peroxide, and gelatin making. | | Originator | Schering AG (Germany) | | Uses | A synthetic analogue of Prostacyclin (PGI2) used to treat pulmonary arterial hypertension (PAH), scleroderma, Raynaud''s phenomenon and ischemia. It acts through elevation of cAMP by binding to the prostacyclin receptor (IP receptor). Iloprost inhibits the ADP, thrombin, and collagen-induced aggregation of human platelets with an ED50 of about 13 nM. In whole animals, iloprost acts as a vasodilator, hypotensive, antidiuretic, and prolongs bleeding time. | | Definition | ChEBI: A carbobicyclic compound that is prostaglandin I2 in which the endocyclic oxygen is replaced by a methylene group and in which the (1E,3S)-3-hydroxyoct-1-en-1-yl side chain is replaced by a (3R
/stereo>)-3-hydroxy-4-methyloct-1-en-6-yn-1-yl group. A synthetic analogue of prostacyclin, it is used as the trometamol salt (generally by intravenous infusion) for the treatment of peripheral vascular disease and pulmonary hypertension. | | Brand name | Ventavis (Schering);Iiomedin. | | Health Hazard | Exposures to quinine vapor are highly irritating to the eyes and may be followed by corneal
opacities, structural changes in the cornea, and loss of vision. Solid quinone may
produce discoloration, severe irritation, swelling, and form papules and vesicles. | | Biological Activity | Prostacyclin (PGI 2 ) analog that binds with high affinity to IP, EP 1 and EP 3 receptors (K i values are 11, 11, 56, 284, 619, 1035, 1870 and 6487 nM for IP, EP 1 , EP 3 , EP 4 , FP, DP, EP 2 and TP receptors respectively). Inhibits platelet aggregation induced by collagen, thrombin and ADP (IC 50 values are 0.24, 0.71 and 1.07 nM respectively). | | Biochem/physiol Actions | Iloprost is used to treat children with PH (pulmonary hypertension) after the surgery to clear CHD (congenital heart disease). In transplant patients, this prostacyclin analogue is used to inhibit renal dysfunction. Iloprost performs various functions like vasodilatation and cytoprotection. It has higher stability than prostacyclin. |
| | ILOPROST Preparation Products And Raw materials |
|