| Company Name: |
Merck KGaA
|
| Tel: |
21-20338288 |
| Email: |
ordercn@merckgroup.com |
| Products Intro: |
Product Name:Dinitrogen trioxide (gas) (unlabelled N2O3)
CAS:10544-73-7
|
|
| | dinitrogen trioxide Basic information |
| Product Name: | dinitrogen trioxide | | Synonyms: | dinitrogen trioxide;NITROGENSESQUIOXIDE;Nitrogen trioxide: (Dinitrogen trioxide);1,3-Diaza-2,4,5-trioxabicyclo[1.1.1]pentane;Chebi:29799;o2Nno;Trioxido-1kappa(2)o,2kappao-dinitrogen(N--N);Dinitrogen trioxide (gas) (unlabelled N2O3) | | CAS: | 10544-73-7 | | MF: | N2O3 | | MW: | 76.011 | | EINECS: | 2341285 | | Product Categories: | | | Mol File: | 10544-73-7.mol | 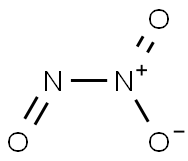 |
| | dinitrogen trioxide Chemical Properties |
| Melting point | -103.15°C | | Boiling point | 2°C (estimate) | | density | 1.400 | | solubility | reacts with H2O | | form | gas | | color | blue solid or liquid
(low temperature) | | EPA Substance Registry System | Nitrogen trioxide (10544-73-7) |
| RIDADR | 2421 | | HazardClass | 2.3 |
| | dinitrogen trioxide Usage And Synthesis |
| Chemical Properties | blue liquid; used as an oxidant in special fuel systems [HAW93] | | Definition | ChEBI: Dinitrogen trioxide is a nitrogen oxide. | | General Description | A blue liquid with a sharp, unpleasant chemical odor. Density 1.447 g / cm3. Low-boiling (boiling point 3.5°C) and held as a liquid by compression. Partially dissociates into NO and NO2. Strong irritant to skin, eyes and mucous membranes. Vapors very toxic by inhalation. Used in special purpose fuels. Under prolonged exposure to intense heat the container may rupture violently and rocket. | | Reactivity Profile | dinitrogen trioxide is an oxidizing agent. Nonflammable but may cause fires when mixed with combustible materials. Reacts with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). Catalyzes ignition of phosphine gas [Edin. Roy. Soc. 13:88. 1935]. A mixture with caprolactam dissolved in acetic acid is explosive unless effectively cooled. Incompatible with phosphorus, or other reduced materials Reactivity likely to resemble that of nitrogen dioxide and nitrogen tetraoxide.. . | | Health Hazard | TOXIC; may be fatal if inhaled or absorbed through skin. Fire will produce irritating, corrosive and/or toxic gases. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Runoff from fire control may cause pollution. | | Fire Hazard | Substance does not burn but will support combustion. Vapors from liquefied gas are initially heavier than air and spread along ground. These are strong oxidizers and will react vigorously or explosively with many materials including fuels. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react violently with air, moist air and/or water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. |
| | dinitrogen trioxide Preparation Products And Raw materials |
|