| Company Name: |
Nanjing Xinmeihe Pharmaceutical Co., Ltd.
|
| Tel: |
025-57672321 13739186949 |
| Email: |
2548785915@qq.com |
| Products Intro: |
Product Name:Azetirelin
CAS:95729-65-0
Purity:98% HPLC Package:1g,5g,10g,50g,500g
|
| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
15002134094 |
| Email: |
marketing@targetmol.cn |
| Products Intro: |
Product Name:Azetirelin
CAS:95729-65-0
Purity:99.72% Package:50mg/RMB 13800;25mg/RMB 10600;100mg/RMB 17500
|
| Company Name: |
United States Biological
|
| Tel: |
800.520.3011 or 781.639.5092 |
| Email: |
chemicals@usbio.net |
| Products Intro: |
Product Name:Azetirelin
CAS:95729-65-0
|
Azetirelin manufacturers
- Azetirelin
-

- $1980.00 / 50mg
-
2024-11-18
- CAS:95729-65-0
- Min. Order:
- Purity: 99.72%
- Supply Ability: 10g
|
| | Azetirelin Basic information |
| Product Name: | Azetirelin | | Synonyms: | 1-[Nα-[[(S)-4-Oxo-2-azetidinyl]carbonyl]-L-histidyl]-L-prolinamide;Nα-[(S)-4-Oxo-2-azetidinyl]carbonyl-L-His-L-Pro-NH2;Nα-[[(S)-4-Oxo-2-azetidinyl]carbonyl]-L-His-L-Pro-NH2;YM-14673;Azetirelin;Nα-[((S)-4-oxo-2-azetidinyl)carbonyl]-L-histidyl-L-prolineamide;Azetirelin (~85%);Azetirelina | | CAS: | 95729-65-0 | | MF: | C15H20N6O4 | | MW: | 348.36 | | EINECS: | | | Product Categories: | | | Mol File: | 95729-65-0.mol | 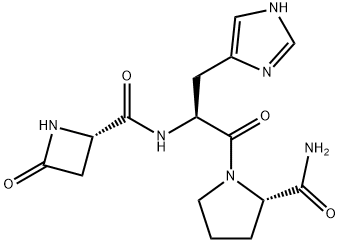 |
| | Azetirelin Chemical Properties |
| Melting point | >125°C (dec.) | | Boiling point | 997.1±65.0 °C(Predicted) | | density | 1.469±0.06 g/cm3(Predicted) | | storage temp. | -20°C Freezer, Under inert atmosphere | | solubility | DMSO (Slightly), Methanol (Slightly), Water (Very Slightly, Heated) | | form | Solid | | pka | 13.98±0.40(Predicted) | | color | Off-White |
| | Azetirelin Usage And Synthesis |
| Originator | Azetirelin,ZYF Pharm Chemical | | Uses | Azetirelin is a thyrotropin-releasing hormone (TRH) analogue. It demonstrated neuroprotective activity in rats. | | Manufacturing Process | In 13 ml of DMF was dissolved 826.0 mg of L-histidyl-L-prolinamide 2-
hydrobromide and then 2 ml of a DMF solution of 405.0 mg of triethylamine
was added to the solution under ice-cooling. After maintaining 30 min under
ice-cooling, the precipitates thus formed were filtered off to provide L-histidyl-
L-prolinamide.
In 10 ml of DMF was dissolved 230.0 mg of (S)-2-azetidinone-4-carboxylic
acid and then 351.0 mg of N-hydroxy-1,2,3-benzotriazole and 453.0 mg of
dicyclohexylcarbodiimide were added to the solution under ice-cooling. Then,
after stirring the mixture for 15 min, the reaction was maintained for 15 min
at room temperature. The reaction mixture was ice-cooled again and 15 ml of
a DMF solution of foregoing L-histidyl-L-prolinamide was added to the reaction
mixture followed by reaction overnight at 0°C. The precipitates thus formed
were filtered off, the filtrate was concentrated to dryness, the residue was
dissolved in 10 ml of chloroform-methanol (4:1) and subjected to silica gel
column chromatography. The eluates by chloroform-methanol (7:3) were
collected and concentrated to dryness to provide 509.0 mg of crude product.
When the product was subjected to silica gel column chromatography again
and eluted by a mixture of chloroform, methanol, and aqueous ammonia
(40:10:1) to provide 394.0 mg of pure Nepsilon-[(S)-2-azetidinone-4-carbonyl]-
L-histidyl-L-prolinamide, melting point 183°-185°C (crystallization from a
small amount of methanol). | | Therapeutic Function | TRH analogue |
| | Azetirelin Preparation Products And Raw materials |
|