|
|
| | (4-Bromophenyl)-tert-butoxycarbonylaminoacetic acid methyl ester Basic information |
| Product Name: | (4-Bromophenyl)-tert-butoxycarbonylaminoacetic acid methyl ester | | Synonyms: | (4-BROMO-PHENYL)-TERT-BUTOXYCARBONYLAMINO-ACETIC ACID METHYL ESTER;Methyl 2-(4-broMophenyl)-2-{[(tert-butoxy)carbonyl]aMino}acetate;Methyl 2-((4-broMophenyl)(tert-butoxycarbonyl)aMino)acetate;Methyl 2-(Boc-amino)-2-(4-bromophenyl)acetate;2-amino-2-(4-bromophenyl)propanedioic acid O3-tert-butyl ester O1-methyl ester;methyl 2-(4-bromophenyl)-2-[(2-methylpropan-2-yl)oxycarbonylamino]acetate;Benzeneacetic acid, 4-bromo-α-[[(1,1-dimethylethoxy)carbonyl]amino]-, methyl ester;Methyl 2-(4-Bromophenyl)-2-(Boc-amino)acetate | | CAS: | 709665-73-6 | | MF: | C14H18BrNO4 | | MW: | 344.21 | | EINECS: | | | Product Categories: | | | Mol File: | 709665-73-6.mol | 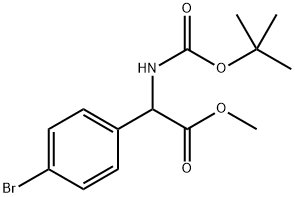 |
| | (4-Bromophenyl)-tert-butoxycarbonylaminoacetic acid methyl ester Chemical Properties |
| Boiling point | 429.9±40.0 °C(Predicted) | | density | 1.354±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C | | pka | 10.53±0.46(Predicted) | | Appearance | White to off-white Solid |
| | (4-Bromophenyl)-tert-butoxycarbonylaminoacetic acid methyl ester Usage And Synthesis |
| Synthesis | General procedure for the synthesis of methyl 2-(4-bromophenyl)-2-(tert-butoxycarbonyl)aminoacetate from di-tert-butyl dicarbonate and methyl 2-amino-2-(4-bromophenyl)acetate:
1. Synthesis of intermediate 1: 2-Amino-2-(4-bromophenyl)acetic acid (3 g) was suspended in a mixed solution of concentrated hydrochloric acid (10 ml) and methanol (10 ml). The reaction mixture was stirred at room temperature for 2 days. A white precipitate was collected by filtration and dried to give methyl 2-amino-2-(4-bromophenyl)acetate as a white powder (49% yield).
2. 2-Amino-2-(4-bromophenyl)acetic acid methyl ester (18 g) was suspended in acetone (15 ml), 1M Na2CO3 solution (15 ml) was added, followed by di-tert-butyl dicarbonate (11 equiv). The mixture was stirred at room temperature for 4 days. The precipitate was collected by filtration and recrystallized from ethyl acetate to give methyl 2-(4-bromophenyl)-2- tert-butoxycarbonylaminoacetate as a colorless solid (38% yield).
3. 2-(4-Bromophenyl)-2- tert-butoxycarbonylaminoacetic acid methyl ester (3.45 g) was dissolved in tetrahydrofuran (52 ml) and water (8 ml). Potassium acetate (1 eq.), 1,3-diphenylphosphonitrile (102 eq.) and Pd(OAc)2 (0.04 eq.) were added. The mixture was stirred at 50°C under carbon monoxide pressure of 150 atm. After completion of the reaction, it was cooled to room temperature, filtered and dried over MgSO4. The solvent was removed under reduced pressure and the residue was purified by fast chromatography (DCM/MeOH 95/5 containing 0.5% formic acid) to afford 4-(tert-butoxycarbonylamino-methoxycarbonyl-methyl)-benzoic acid as a light yellow powder (57% yield).
4. 4-(Boc-amino-methoxycarbonyl-methyl)-benzoic acid (175 g) was dissolved in DMF (10 ml), diisopropylethylamine (5 eq.), HOBt (1 eq.) and TBTU (13 eq.) were added. The mixture was stirred at room temperature for 30 min and then 4-aminopyridine (1 eq.) was added. Stirring was continued for 2 days. The solvent was removed under reduced pressure and the residue was dissolved in DCM (100 ml) and washed with 1N sodium bicarbonate solution (3 x 100 ml). The organic layer was dried with MgSO4, filtered and the solvent was removed under reduced pressure. The residue was purified by fast chromatography (DCM/MeOH 99/1 to 95/5) to afford tert-butoxycarbonylamino-[4-(pyridin-4-ylcarbamoyl)-phenyl]-acetic acid methyl ester as a yellow powder (56% yield).
5. Lithium hydroxide (12 eq.) was added to a solution of tert-butoxycarbonylamino-[4-(pyridin-4-ylcarbamoyl)-phenyl]-acetic acid methyl ester (712 mg) in water/acetone (9:1, 10 ml). The mixture was stirred at room temperature for 18 hours before adding lithium hydroxide (24 equiv.) and continuing to stir for 24 hours. After completion of the reaction, it was neutralized with 1N HCl to pH=7. The aqueous layer was extracted with ethyl acetate (3 x 30 ml), the organic layers were combined, dried over MgSO4, filtered, and the solvent was removed under reduced pressure to afford tert-butoxycarbonylamino-[4-(pyridin-4-ylcarbamoyl)-phenyl]-acetic acid as a yellow powder (56% yield).
1H NMR (300 MHz, DMSO-d6) δ 1.39 ppm (s, 9H), 5.20 ppm (d, 1H, J=8.0 Hz), 7.56 ppm (d, 2H, J=8.3 Hz), 7.77 ppm (d, 2H, J=6.3 Hz), 7.92 ppm (d, 2H, J=8.3 Hz), 8.47 ppm (d, 2H, J=6.3 Hz), 10.60 ppm (s, 1H). | | References | [1] Patent: WO2007/6547, 2007, A1. Location in patent: Page/Page column 36-37
[2] European Journal of Medicinal Chemistry, 2016, vol. 110, p. 43 - 64
[3] Patent: WO2017/8101, 2017, A1. Location in patent: Paragraph 0047 |
| | (4-Bromophenyl)-tert-butoxycarbonylaminoacetic acid methyl ester Preparation Products And Raw materials |
|