|
|
| | Ethyl 1,1,2,2-tetrafluoroethyl ether Basic information |
| Product Name: | Ethyl 1,1,2,2-tetrafluoroethyl ether | | Synonyms: | ether,ethyl1,1,2,2-tetrafluoroethyl;1,1,2,2-Tetrafluoro ethyl ether;3-Oxa-1,1,2,2-tetrafluoropentane, 1-Ethoxy-1,1,2,2-tetrafluoroethane;Ethane,1-ethoxy-1,1,2,2-tetrafluoro-;HYDROFLUOROETHER(HFE-374);1-ethoxy-1,1,2,2-tetrafluoro-ethan;1-ethoxy-1,1,2,2-tetrafluoroethane;374pcebetagamma | | CAS: | 512-51-6 | | MF: | C4H6F4O | | MW: | 146.08 | | EINECS: | | | Product Categories: | | | Mol File: | 512-51-6.mol | 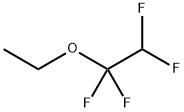 |
| | Ethyl 1,1,2,2-tetrafluoroethyl ether Chemical Properties |
| Hazard Codes | F,Xi | | Risk Statements | 11 | | Safety Statements | 16-23-33 | | RIDADR | 3271 | | RTECS | KI3590000 | | Hazard Note | Flammable | | HazardClass | 3 | | PackingGroup | II | | HS Code | 2909199090 | | Toxicity | LC inhalation in rat: > 30000ppm/4H |
| Provider | Language |
|
ALFA
| English |
| | Ethyl 1,1,2,2-tetrafluoroethyl ether Usage And Synthesis |
| Description |
Ethyl 1,1,2,2-tetrafluoroethyl ether (ETFE) is a fluorinated linear ether with several fundamental properties that make it useful in batteries. First, ETFE is miscible with many polar organic solvents used in electrolyte formulations, which improves the ionic conductivity of the battery. Additionally, ETFE has a relatively low viscosity (486 mPa?s at 23 °C) and a low boiling point (58 °C), which make it a versatile diluent for low-temperature applications.
| | Uses |
Ethyl 1,1,2,2-tetrafluoroethyl ether (ETFE) is a highly effective additive in lithium-ion and sodium-ion batteries for improving battery performance, increasing safety, and reducing flammability. ETFE is a versatile co-solvent with high miscibility, which allows it to be blended with other solvents to achieve high ionic conductivities. It is also a good SEI-forming electrolyte additive, stabilizing the Na electrode surface and enhancing the stability of high-voltage batteries. ETFE is particularly useful as an electrolyte co-solvent in lithium-sulfur (Li-S) batteries. Studies have shown that ETFE-containing electrolytes improve the stability of the electrolyte/anode interface and enhance the cycling, rate, and self-discharging of the Li-S cell more than glyme-based cosolvents or unblended solvents.
|
| | Ethyl 1,1,2,2-tetrafluoroethyl ether Preparation Products And Raw materials |
|