| Company Name: |
AdooQ BioScience, LLC
|
| Tel: |
+1 (866) 930-6790 |
| Email: |
info@adooq.com |
| Products Intro: |
Product Name:Chaetominine
CAS:918659-56-0
Purity:98% Package:5mg;10mg;25mg;50mg;100mg;250mg;500mg;1g
|
| Company Name: |
AdooQ Bioscience CHINA
|
| Tel: |
025-58849295 18951903616; |
| Email: |
info@adooq.cn |
| Products Intro: |
Product Name:Chaetominine
CAS:918659-56-0
Purity:0.98 Package:1mg;5mg;10mg;25mg;50mg;100mg
|
|
| | Chaetominine Basic information |
| Product Name: | Chaetominine | | Synonyms: | Chaetominine;(2S,4R,5aS,9cS)-4,5,5a,9c-Tetrahydro-5a-hydroxy-2-methyl-4-(4-oxo-3(4H)-quinazolinyl)-3H-2a,9b-diazacyclopenta[jk]fluorene-1,3(2H)-dione;Chaetominine
(-)-Chaetominine;(2S,2a1S,4R,5AS)-5a-hydroxy-2-methyl-4-(4-oxoquinazolin-3(4H)-yl)-2a1,4,5,5a-tetrahydro-1H-2a;(-)-CHAETOMININE;CS-493;3H-2a,9b-Diazacyclopenta[jk]fluorene-1,3(2H)-dione, 4,5,5a,9c-tetrahydro-5a-hydroxy-2-methyl-4-(4-oxo-3(4H)-quinazolinyl)-, (2S,4R,5aS,9cS)-;(–)-Chaetominine | | CAS: | 918659-56-0 | | MF: | C22H18N4O4 | | MW: | 402.4 | | EINECS: | 604-604-1 | | Product Categories: | | | Mol File: | 918659-56-0.mol | 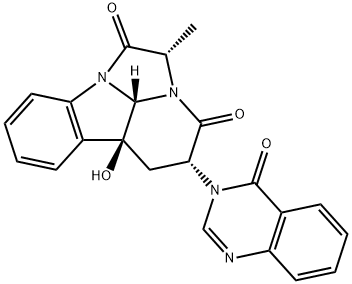 |
| | Chaetominine Chemical Properties |
| Melting point | 164℃ | | density | 1.63 | | storage temp. | Store at -20°C | | solubility | Soluble in DMSO | | form | A solid |
| | Chaetominine Usage And Synthesis |
| Description | (–)-Chaetominine is a cytotoxic alkaloid originally isolated from Chaetomium sp. IFB-E015. It inhibits the growth of K562 leukemia and SW1116 colon cancer cells (IC50s = 20 and 28 nM, respectively). (–)-Chaetominine induces apoptosis of K562 cells via upregulation of the Bax/Bcl-2 ratio, decreasing mitochondrial membrane potential, inducing mitochondrial cytochrome C release, and activation of caspase-3 and caspase-9. It also decreases doxorubicin efflux mediated by multidrug resistance-associated protein 1 (MRP1) and restores sensitivity to doxorubicin in resistant K562 cells. | | Definition | ChEBI: Chaetominine is an organic heterotetracyclic compound that consists of 4,5,5a,9c-tetrahydro-3H-2a,9b-diazacyclopenta[jk]fluorene-1,3(2H)-dione substituted by a hydroxy group at position 5, a methyl group at position 2 and a 4-oxoquinazolin-3(4H)-yl group at position 4 (the 2S,4R,5aS,9cS stereoisomer). It is a cytotoxic alkaloid isolated from the endophytic fungus Chaetomium. It has a role as a metabolite. It is an organic heterotetracyclic compound, a member of quinazolines, a lactam and an indole alkaloid. |
| | Chaetominine Preparation Products And Raw materials |
|