- LDE-225 Diphosphate
-

- $1.00 / 1g
-
2020-01-13
- CAS:1218778-77-8
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 200kg
|
| | LDE-225 Diphosphate Basic information |
| Product Name: | LDE-225 Diphosphate | | Synonyms: | LDE225 Diphosphate, >=98%;Erismodegib Diphosphate, NVP-LDE225 (diphosphate salt);ErisModegib Diphosphate;LDE 225 Diphosphate;LDE225 (Diphosphate);LDE-225 Diphosphate;NVP-LDE 225 Diphosphate;LDE 225 phosphate | | CAS: | 1218778-77-8 | | MF: | C26H32F3N3O11P2 | | MW: | 681.4885116 | | EINECS: | | | Product Categories: | | | Mol File: | 1218778-77-8.mol | 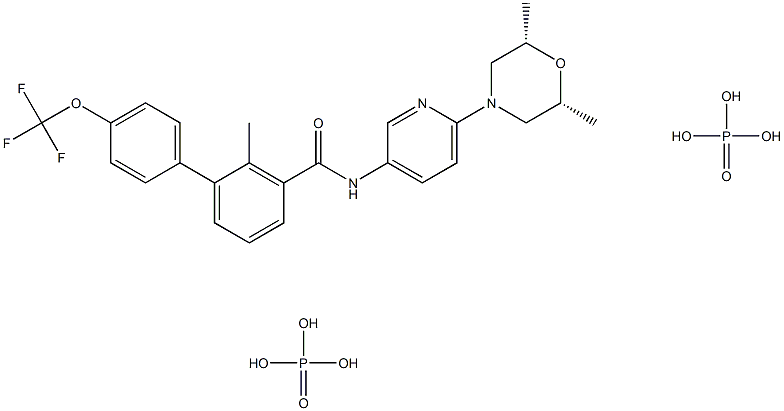 |
| | LDE-225 Diphosphate Chemical Properties |
| storage temp. | Store at -20°C | | solubility | ≥27.85 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH | | form | White solid. | | color | White to off-white |
| | LDE-225 Diphosphate Usage And Synthesis |
| Description | Sonidegib phosphate (XXIX),
an orally bioavailable, small molecule smoothened (SMO)
receptor antagonist developed by Novartis, was approved in
2015 in Switzerland, the U.S., and the EU for the treatment of
adult patients with advanced or locally advanced basal cell
carcinoma (BCC). BCC is the most frequently diagnosed
skin cancer, constituting 80% of all nonmelanoma skin
cancers. While most BCCs can be treated through surgery
or radiation therapy, some patients (<10%) have more
advanced tumors for which surgery may be contraindicated
or impractical; treatment options for these patients are limited.
SMO, a G-protein coupled receptor-like protein (GPCR), is a
key regulator in the hedgehog (Hh) pathway, which is activated
in a number of tumors including BCC. In a multicenter
clinical trial for sonidegib, an objective response rate of 43%
was observed for patients with locally advanced BCC (dosing at
200 mg once daily), with sustained clinically meaningful
responses based on an 18-month analysis. Sonidegib joins
vismodegib as marketable treatments of BCC. Vismodegib,
which was approved in 2012 as a first-in-class SMO receptor
antagonist, represented the first Hedgehog signaling pathway
targeting agent to gain FDA approval. | | Uses | NVP-LDE225 Diphosphate Salt is a potent, selective, and orally bioavailable Smoothened (Hedgehog Signaling Pathway) antagonist. | | Definition | ChEBI: A phosphate salt obtained by reaction sonidegib with two equivalent of phosphoric acid. Used for treatment of locally advanced basal cell carcinoma. | | Synthesis | The synthesis was initiated with an SNAr reaction involving commercial 2-chloro-
5-nitropyridine (226) and cis-dimethylmorpholine (227)
followed by subsequent nitro reduction via hydrogenation to
provide amine 228. The crude amine was coupled directly to 3-
bromo-2-methylbenzoic acid (229) in an amide bond-forming
reaction to construct 230 in 77% yield over three steps. The
resultant bromide was coupled to 4-(trifluoromethoxy)-
phenylboronic acid (231) under Suzuki conditions to give
rise to sonidegib as the freebase. Finally, treatment with 85%
aqueous phosphoric acid in acetonitrile generated sonidegib
phosphate (XXIX) in good yield. 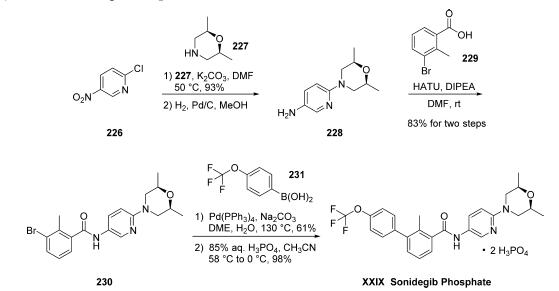
| | in vitro | lde225 was found to selectively bind to the hedgehog (hh)-ligand cell surface receptor smo, which might result in the suppression of the hh signaling pathway and, therefore, the inhibition of tumor cells in which this pathway was abnormally activated [1]. | | in vivo | in the subcutaneous medulloblastoma allograft mouse model, lde225 demonstrated dose-related antitumor activity after 10 days of oral administration of a suspension. at a dose of 5 mg/kg/ day qd, lde225 inhibited tumor growth significantly, corresponding to a t/c value of 33%. when dosed at 10 and 20 mg/kg/day qd, lde225 afforded 51 and 83% regression, respectively [1]. | | IC 50 | 1.3 and 2.5 nm for mouse and human smo | | references | [1] shifeng pan,xu wu,jiqing jiang et al. discovery of nvp-lde225, a potent and selective smoothened antagonist. acs med chem lett. 2010 jun 10; 1(3): 130–134.
[2] rodon j,tawbi ha,thomas al,stoller rg,turtschi cp,baselga j,sarantopoulos j,mahalingam d,shou y,moles ma,yang l,granvil c,hurh e,rose kl,amakye dd,dummer r,mita ac. a phase i, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor sonidegib (lde225) in patients with advanced solid tumors. clin cancer res.2014 apr 1;20(7):1900-9. |
| | LDE-225 Diphosphate Preparation Products And Raw materials |
|