- COBALT(III) OXIDE BLACK
-

- $0.00 / 25KG
-
2025-03-21
- CAS:1308-04-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
|
| Product Name: | COBALT(III) OXIDE BLACK | | Synonyms: | dicobalttrioxide;COBALT(III) OXIDE R. G., FOR THE DETERMI NATION OF SULFUR;COBALT(III) OXIDE BLACK, PURE;Cobalt(Ⅱ,Ⅲ)oxide;Cobaltic oxide monohydrate;BLACKCOBALTICOXIDE;cobalt(iii)oxide;cobaltoxide(co2o3) | | CAS: | 1308-04-9 | | MF: | Co2O3 | | MW: | 165.86 | | EINECS: | 215-156-7 | | Product Categories: | Inorganics | | Mol File: | 1308-04-9.mol | 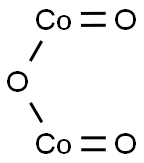 |
| | COBALT(III) OXIDE BLACK Chemical Properties |
| Melting point | 895 °C (dec.)(lit.) | | density | 6.11 g/mL at 25 °C(lit.) | | storage temp. | Inert atmosphere,Room Temperature | | solubility | insoluble in H2O; soluble in concentrated acid solutions | | form | powder | | color | gray-black | | Water Solubility | insoluble H2O; soluble conc acids [HAW93] | | EPA Substance Registry System | Cobalt oxide (Co2O3) (1308-04-9) |
| Hazard Codes | Xn | | Risk Statements | 36/37/38-40-43-22 | | Safety Statements | 26-36/37/39-36/37 | | WGK Germany | 1 | | RTECS | GG2500000 | | Toxicity | mouse,LD50,subcutaneous,2064mg/kg (2064mg/kg),Zhurnal Vsesoyuznogo Khimicheskogo Obshchestva im. D.I. Mendeleeva. Journal of the D.I. Mendeleeva All-Union Chemical Society. Vol. 19, Pg. 186, 1974. |
| | COBALT(III) OXIDE BLACK Usage And Synthesis |
| Uses | COBALT(III) OXIDE BLACK is used as a pigment; for glazing porcelain and pottery; and for coloring enamels.
| | Preparation | COBALT(III) OXIDE BLACK is prepared by heating cobalt compounds at low temperatures in air.
| | Reactions | Heating with hydrogen, carbon or carbon monoxide reduces the oxide to cobalt metal.
Co2O3 + 3H2→ 2Co + 3H2O
Co2O3 + 3CO→ 2Co + 3CO
2Co2O3 + 3C→ 4Co + 3CO2
Strong heating in air converts cobalt(III) oxide to tricobalt tetroxide.
Reactions with mineral acids produce their Co3+ salts:
Co2O3 + 6HCl → 2CoCl3 + 3H2O
| | Description | Neither the oxide Co2O3 nor the hydroxide Co(OH)3 is definitely established. The oxidation of cobalt(II) hydroxide in aqueous suspension with, for example, peroxides, or the destruction of a cobalt(III) complex with alkali gives a brown or black powder Co2O3.aq which upon drying at 150° yields the monohydrate Co2O3.H2O; this is probably best formulated as CoO(OH). When heated further in attempts to dehydrate it, the "monohydrate" begins to evolve oxygen (as well as water) at 300° with the formation of black Co3O4. When heated to 100° in air cobalt(II) hydroxide is converted to dark brown CoO(OH). | | Chemical Properties | Steel-gray or black powder.Soluble in concentrated acids; insoluble in water. | | Physical properties | Grayish black powder; density 5.18 g/cm3; decomposes at 895°C; insoluble in water; soluble in concentrated mineral acids. | | Uses | Pigment, coloring enamels, glazing pottery. | | Uses | Cobaltic oxide (Co2O3) is also known as cobalt oxide or cobalt black. It is dark gray to black
and is used in pigments, ceramic glazes, and semiconductors. | | Preparation | Cobalt(III) oxide is prepared by heating cobalt compounds at low temperatures in air. | | Definition | Sometimes incorrectly called cobalt peroxide. | | Safety Profile | : Moderately toxic by
intraperitoneal and subcutaneous routes.
Questionable carcinogen. Violent reaction
with hydrogen peroxide. The oxide increases
the sensitivity of nitroalkanes (e.g.,
nitromethane, nitroethane, and 1
nitropropane) to heat or detonation. See
also COBALT COMPOUNDS. |
| | COBALT(III) OXIDE BLACK Preparation Products And Raw materials |
|