- Pemetrexed disodium
-

- $0.00 / 1g/Bag
-
2025-03-29
- CAS:150399-23-8
- Min. Order: 1g
- Purity: 99%min
- Supply Ability: 10kg
- Pemetrexed disodium
-

- $0.00 / 25kg
-
2025-03-21
- CAS:150399-23-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000kg
- Pemetrexed disodium
-

- $51.00 / 50mg
-
2024-11-16
- CAS:150399-23-8
- Min. Order:
- Purity: 99.92%
- Supply Ability: 10g
|
| | Pemetrexed Disodium Chemical Properties |
| Melting point | 254-258°C (dec.) | | storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C | | solubility | Methanol, Water | | form | Solid | | color | Off-White | | Stability: | Hygroscopic | | InChIKey | UTEALKYVANXUSG-NVZXTOETNA-N | | SMILES | C(C1=CNC2NC(N)=NC(=O)C1=2)CC1C=CC(C(=O)N[C@H](C(=O)O)CCC(=O)O)=CC=1.[NaH] |&1:20,r| | | CAS DataBase Reference | 150399-23-8(CAS DataBase Reference) |
| | Pemetrexed Disodium Usage And Synthesis |
| Description | Pemetrexed disodium (marketed as ALIMTA®, “pemetrexed”) is a novel, multi-targeted antifolate. It suppresses tumor growth by impeding both DNA synthesis and folate metabolism. Pemetrexed disodium has demonstrated promising clinical activity in a wide variety of solid tumors, including non-small cell lung, breast, mesothelioma, colorectal, pancreatic, gastric, bladder, cervix, and head and neck.
Pemetrexed disodium is approved to be used alone or with other drugs to treat malignant pleural mesothelioma in patients who cannot be treated with surgery and non-small cell lung cancer (certain types) in patients whose disease is locally advanced or has metastasized (spread to other parts of the body). Pemetrexed disodium is also being studied in the treatment of other types of cancer.
| | References | [1] https://www.cancer.gov/about-cancer/treatment/drugs/pemetrexeddisodium
[2] Axel-R. Hanauske, V. Chen P. Paoletti, C. Niyikiza (2001) Pemetrexed Disodium: A Novel Antifolate Clinically Active Against Multiple Solid Tumors, The Oncologist, 6, 363-373
| | Description | Pemetrexed, a pyrrolo[2,3-d]pyrimidine-based antifolate that disrupts cell replication
by inhibiting multiple folate-dependent metabolic processes, was initially developed
and launched in the US for the treatment of malignant pleural
mesothelioma in conjunction with cisplatin. Patients who are not candidates for
surgery may benefit from this combination therapy. Clinical data demonstrated that
the median overall survival time increased to 12.1 months, compared with 9.3
months for patients receiving cisplatin alone. In August of 2004, the FDA also
approved pemetrexed as a second-line treatment of non-small-cell lung cancer
(NSCLC). While median survival is comparable to the standard second-line treatment
docetaxel, the improved toxicity profile (significant reduction in neutropenia)
accelerated the approval for NSCLC. Its effectiveness as an anticancer drug is
derived from its ability to gain internal cell access via the reduced folate carrier and
membrane folate binding protein transport systems. Once inside, pemetrexed undergoes
polyglutamation, and the resultant polyglutamate forms (predominantly
the pentaglutamate) inhibit the folate-dependent enzymes thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide
formyltransferase (GARFT). Against recombinant human TS, pemetrexed has a
Ki of 109nM while the triglutamate and pentaglutamate forms have Ki values of
1.6nM and 1.3 nM, respectively. All forms of pemetrexed display similar potency
against recombinant human DHFR (7 nM), but the pentaglutamate form is significantly
more potent against recombinant murine GARFT than the parent
(Ki=65nM versus 9.3μM). The selectivity of pemetrexed may be explained by the
fact that polyglutamation is more likely to occur in cancer cells compared to normal
cells while its prolonged duration of action may be attributed to decreased cellular
efflux of the polyglutamate forms. While several different routes have provided
pemetrexed, one of the most efficient exploits the propensity of 2,6-diamino-3Hpyrimidin-
4-one to undergo Michael additions at its unsubstituted C-5 position.
Using ethyl 4-(4-nitrobut-3-enyl)benzoate as the Michael acceptor, the resulting
adduct is then converted to the ultimate precursor for glutamyl coupling via a onepot,
three-step process (Nef reaction to transform the nitro to the aldehyde, intramolecular
condensation to afford the pyrrole, and saponification of the ethyl ester). A
typical treatment regimen involves intravenous administration of pemetrexed, infused
over ten minutes, at a dose of 500mg/m2 followed by a thirty minute wash-out period
and then cisplatin intravenously over two hours at a dose of 75mg/m2. Both drugs are
given on Day 1 of a 21-day cycle. In order to reduce treatment-related hematological
and GI toxicity, patients are instructed to take folic acid and vitamin B12 as a prophylactic
measure. Pretreatment with a corticosteroid is also recommended to prevent
possible skin rashes. Pemetrexed is primarily excreted intact in the urine, with 70–90%
of the dose being recovered within 24 hours of administration. The half-life of pemetrexed
is 3.5 hours in patients with normal renal function, and the total systemic
clearance is 91.8mL/min. As expected, clearance decreases as renal impairment increases.
The drug’s plasma protein binding is 81%, and it has a steady state volume of
distribution of 16.1 L. The pharmacokinetics of pemetrexed is linear with dose and
remains unchanged over multiple treatment cycles. While in vitro studies suggest that
pemetrexed would not interfere with drugs metabolized by CYP3A4, CYP2D6,
CYP2C9, and CYP1A2, ibuprofen (400mg q.d.) does reduce pemetrexed clearance by
20%. Caution should, therefore, be taken when administering pemetrexed concurrently
with ibuprofen to patients with renal insufficiency and should not be given at
all to patients whose creatinine clearance is <45mL/min.
. | | Chemical Properties | Crystalline Solid | | Originator | Eli Lilly (US) | | Uses | Multitargeted antifolate; inhibits thymidylate synthase as well as other folate dependent enzymes. Antineoplastic. | | Uses | Pemetrexed is a novel antifolate and antimetabolite for TS, DHFR and GARFT with Ki of 1.3 nM, 7.2 nM and 65 nM, respectively | | Definition | ChEBI: An organic sodium salt that is the disodium salt of N-{4-[2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-L-glutamic acid. Inhibits thymidylate synthase (TS), 4
1 dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT). | | Brand name | Alimta (Lilly). | | General Description | The drug is available in a 100-mg sterile vial for IV use. Thedrug appears to be effective against a range of tumors includingmesothelioma, NSCLC, colorectal cancer, bladdercancer, and lung cancer. The mechanism of action involvesinhibition of TS resulting in inhibition of thymidylate andDNA synthesis. This drug is a pyrrolopyrimidine analog offolate with antifolate activity. Resistance can occur by increasedexpression of TS, decreased binding affinity for TS,or decreased drug transport into cells. The drug is administeredonly via the IV route and distributes to all tissues.Cellular activation to the more potent polyglutamated formsoccurs, and the majority of the dose is excreted unchangedin the urine. The drug interaction and toxicity profiles aresimilar to that of methotrexate. | | Mechanism of action | Like methotrexate, it is actively transported into tumor cells through reduced folate carriers and, in polyglutamated form, inhibits the synthesis of pyrimidine and purine-based nucletotides by disrupting folate�dependent metabolic processes . In addition to DHFR, this pyrrolopyrimidine-based inhibitor binds tightly to thymidylate synthase and GAR transformylase. | | Clinical Use | Pemetrexed is a novel multitarget antifolate used by the IV route for the treatment of advanced or metastatic nonsmall cell lung cancer and in combination with cisplatin in malignant pleural mesothelioma. | | Side effects | Patients on pemetrexed must take folate and vitamin B12 supplements to reduce the risk of bone marrow suppression (neutropenia, thrombocytopenia, and anemia) and GI side effects. Pretreatment with corticosteroids can reduce the risk of drug-induced skin rash. Pemetrexed has a half-life of 3.5 hours and is excreted primarily unchanged via the kidneys. Significant cross-resistance has been noted between pemetrexed and other pyrimidine and folate antagonists. | | Synthesis | A number of papers
outlining the syntheses of pemetrexed and related analogs have appeared. A practical and scalable synthetic
route is depicted in Scheme 9. Palladium (0) coupling
of methyl 4-bromobenzoate (57) with 3-butyn-1-ol (58) gave
crystalline 59, which was then reduced over palladium on
carbon in DCM to give alcohol 60. Filtration of the catalyst
afforded a DCM solution of alcohol 60, which was utilized
directly in a TEMPO-catalyzed sodium hypochlorite
oxidation, providing known aldehyde 61 without isolation.
Addition of 5,5-dibromobarbituric acid (DBBA) and
catalytic amount of HBr in acetic acid to the DCM solution
of 61 effected the conversion to a-bromoaldehyde 62. After
aqueous work-up, the solution was concentrated and diluted
with acetonitrile to exchange solvents. Addition of
commercially available 2,4-diamino-6-hydroxypyrimidine
(63), aqueous sodium acetate and heating to 45??C resulted in
cyclic condensation and precipitation of pyrrolo[2,3-
d]pyrimidine 64 from the reaction mixture in 67% yield
based on 60. Saponification of 64 with aqueous sodium
hydroxide followed by acidification afforded the carboxylic
acid derivative 65, which was elaborated to 66 by
chlorodimethoxytriazine active ester coupling method.
Reaction of 65 with 2-chloro-4,6-dimethoxy-1,3,5-triazine
(CDMT) in the presence of N-methylmorpholine in DMF
solution followed by reaction of the resulting dimethoxy-s-triazinyl ester with diethyl L-glutamate afforded crude 66,
which was isolated via crystallization as pTSA salt 67.
Saponification of 67 with aqueous sodium hydroxide
followed by acidification with HCl gave pemetrexed as the
free acid, which was crystallized as disodium salt form. 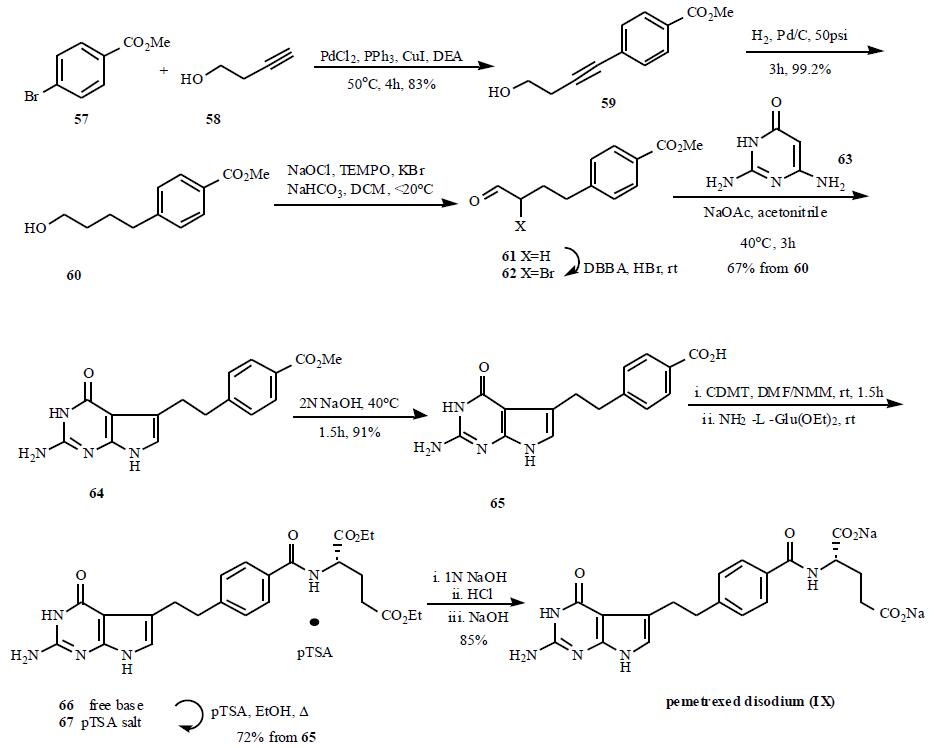
| | target | MEK | ERK | | Drug interactions | Potentially hazardous interactions with other drugs
Antimalarials: antifolate effect increased by
pyrimethamine.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Nephrotoxic agents: may reduce clearance of
pemetrexed - use with caution.
Live vaccines: avoid use; YELLOW
FEVER VACCINE ABSOLUTELY
CONTRAINDICATED. | | Metabolism | Pemetrexed undergoes minimal hepatic metabolism,
and about 70-90% of a dose is eliminated unchanged in
the urine within 24 hours. In vitro studies indicate that
pemetrexed is actively secreted by OAT3 (organic anion
transporter). |
| | Pemetrexed Disodium Preparation Products And Raw materials |
|