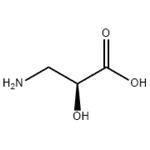
- L-Isoserine
-
- $0.00/ kg
-
2025-04-18
- CAS:632-13-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1T+
- L-Isoserine
-

- $1.00 / 1KG
-
2020-01-05
- CAS:632-13-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100KG
|
| | L-Isoserine Basic information |
| Product Name: | L-Isoserine | | Synonyms: | (S)-ISOSERINE;(S)-2-HYDROXY-B-ALANINE;(S)-2-HYDROXY-BETA-ALANINE;(S)-3-AMINO-2-HYDROXYPROPIONIC ACID;L-ISOSERINE;(s)-2-hydroxy-β-alanine;(S)-LSOSERINE;(2S)-3-AMINO-2-HYDROXY-PROPANOIC ACID | | CAS: | 632-13-3 | | MF: | C3H7NO3 | | MW: | 105.09 | | EINECS: | | | Product Categories: | (intermediate of isepamicin) | | Mol File: | 632-13-3.mol | 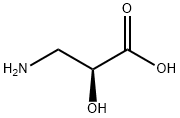 |
| | L-Isoserine Chemical Properties |
| Melting point | 199~201℃ | | Boiling point | 386.6±27.0 °C(Predicted) | | density | 1.415±0.06 g/cm3(Predicted) | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | form | powder to crystal | | pka | pK1:2.72(+1);pK2:9.25(0) (25°C,μ=0.16) | | color | White to Almost white | | Water Solubility | Slightly soluble in water. | | Sensitive | Moisture Sensitive | | InChI | InChI=1S/C3H7NO3/c4-1-2(5)3(6)7/h2,5H,1,4H2,(H,6,7)/t2-/m0/s1 | | InChIKey | BMYNFMYTOJXKLE-REOHCLBHSA-N | | SMILES | C(O)(=O)[C@@H](O)CN | | CAS DataBase Reference | 632-13-3(CAS DataBase Reference) |
| WGK Germany | 3 | | F | 10-21 | | HS Code | 2922500090 |
| | L-Isoserine Usage And Synthesis |
| Uses | It is an important raw material and intermediate used in organic synthesis, pharmaceuticals, agrochemicals and dyestuffs. | | reaction suitability | reaction type: solution phase peptide synthesis | | Biological Activity | Research has found that L-isoserine showed the ability to inhibit Aminopeptidase N (APN) at a half-maximal inhibitory concentration (IC50 ) value of 563 μM, which could serve as a new lead compound for further chemical modification and optimization. In addition, it has been reported that L-isoserine-L-leucine dipeptide shows moderate aminopeptidase B inhibitory activity (IC50 of 140 μM). This evidence indicates that the incorporation of an amino acid to L-isoserine may contribute to its inhibitory activity against APN. L-isoserine induces long-lasting effects by increasing the expression of GAT3 (glial GABA transporte) in peri-infarct regions[1-2]. | | References | [1] Yang K , et al. Synthesis of a novel series of L-isoserine derivatives as aminopeptidase N inhibitors. Journal of Enzyme inhibition and Medicinal Chemistry, 2011; 27: 302-310.
[2] Lie M , et al. GAT3 selective substrate l-isoserine upregulates GAT3 expression and increases functional recovery after a focal ischemic stroke in mice. Journal of Cerebral Blood Flow & Metabolism, 2017; 39. |
| | L-Isoserine Preparation Products And Raw materials |
|