|
|
| | (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINE Basic information | | Reactions |
| Product Name: | (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINE | | Synonyms: | (R R)-(-)-N N''-BIS(3 5-DI-T-BU-SALICYL.) -1 2-CYCLOHEXANEDIA 96+%;N,N'-bis(3,5-dibutylsalicylidene)-1,2-cyclohexanediamine;(1R,2R)-(-)-1,2-Cyclohexanediamino-N,N'-bis(3,5-di-t-butylsalicylidene),98%(R,R)-JacobsenLigand;1R,2R-N,N'-Bis(3,5-di -tert-butylsalicylidene)-1,2-cyclohexanediaMine;2-((E)-((1R,2R)-2-((E)-3,5-di-tert-butyl-2-hydroxybenzylideneaMino)cyclohexyliMino)Methyl)-4,6-di-tert-butylphenol;)-N,N′-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaMine;(R,R)-(-)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine,98%;(R,R)-(-)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine(R,R)-Jacobsen Ligand | | CAS: | 135616-40-9 | | MF: | C36H54N2O2 | | MW: | 546.84 | | EINECS: | | | Product Categories: | Jacobsen Ligand;Chiral Nitrogen;Aromatics;Catalyst;Chelating Agents & Ligands;Intermediates | | Mol File: | 135616-40-9.mol |  |
| | (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINE Chemical Properties |
| Melting point | 205-207 °C(lit.) | | alpha | -315° (c 1, CH2Cl2) | | Boiling point | 619.74°C (rough estimate) | | density | 1.0110 (rough estimate) | | refractive index | 1.5300 (estimate) | | storage temp. | Inert atmosphere,Room Temperature | | solubility | Chloroform (Slightly), Dichloromethane (Slightly) | | pka | 13.06±0.40(Predicted) | | form | Powder | | color | Yellow | | optical activity | [α]20/D 315±15°, c = 1 in methylene chloride | | λmax | 328nm(CH3CN)(lit.) | | BRN | 5464823 | | InChIKey | FYNXDGNCEBQLGC-LQWPWIHBSA-N |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | HS Code | 29222900 |
| | (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINE Usage And Synthesis |
| Reactions | 1. A versatile ligand for asymmetric catalysis.
(a) Conjugate addition of hydrazoic acid to unsaturated imides.
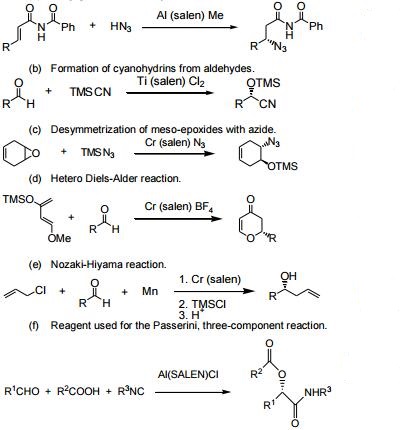 2. Enantioselective conjugate cyanation of unsaturated imides.
2. Enantioselective conjugate cyanation of unsaturated imides.
 3. Cobalt catalyzed enantioselective alpha chlorination and fluorination of beta-ketoesters.
3. Cobalt catalyzed enantioselective alpha chlorination and fluorination of beta-ketoesters.
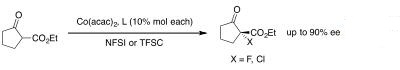
| | Chemical Properties | (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINE is yellow powder | | Uses | Versatile ligand for asymmetric catalysis. Ligand used for preparing Jacobsen′s catalyst suitable for enantioselective epoxidation of olefins lacking functional groups.
Lipases are used industrially for the resolution of chiral compounds and the transesterification production of biodiesel. | | Uses | (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINE is a versatile ligand used in the preparation of Jacobsen's catalyst. | | Biochem/physiol Actions | α-amylase catalyzes the hydrolysis of internal α-1,4-O-glycosidic bonds in starches to release α-anomeric sugars. |
| | (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINE Preparation Products And Raw materials |
|