|
|
| | di(4-t-butylphenyl)iodonium camphorsulfonate Basic information |
| Product Name: | di(4-t-butylphenyl)iodonium camphorsulfonate | | Synonyms: | di(4-t-butylphenyl)iodonium camphorsulfonate;DTBPI-CS;Iodonium, bis[4-(1,1-dimethylethyl)phenyl]-, 7,7-dimethyl-2-...;Iodonium, bis[4-(1,1-dimethyl- ethyl)phenyl]-, 7,7-dimethyl- 2-oxobicyclo[2.2.1]heptane-1-methanesulfonate (1:1);Bis(tert-butylphenyl)iodonium 10-camphorsulfonate;Bis-(4-tert-butylphenyl)-iodonium
10-camphorsulfonate;Iodonium, bis[4-(1,1-dimethylethyl)phenyl]-, 7,7-dimethyl-2-oxobicyclo[2.2.1]heptane-1-methanesulfonate C | | CAS: | 193345-23-2 | | MF: | C30H41IO4S | | MW: | 624.62 | | EINECS: | 682-849-2 | | Product Categories: | | | Mol File: | 193345-23-2.mol |  |
| | di(4-t-butylphenyl)iodonium camphorsulfonate Chemical Properties |
| InChIKey | MDUKBVGQQFOMPC-UHFFFAOYSA-M | | SMILES | [I+](C1=CC=C(C(C)(C)C)C=C1)C1=CC=C(C(C)(C)C)C=C1.C(S([O-])(=O)=O)C12C(C)(C)C(CC1)CC2=O |
| | di(4-t-butylphenyl)iodonium camphorsulfonate Usage And Synthesis |
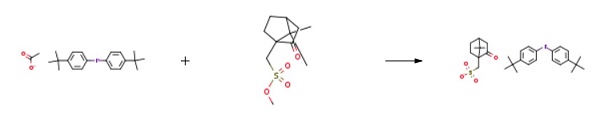
| Synthesis | Ethyl acetate (50 ml) was added to bis(p-tert-butylphenyl)iodonium acetate (467 mg, 1 mmol) and methyl camphorsulfonate (259 mg, 1.05 mmol). The resultant mixture was refluxed for 24 hours while being stirred. The reaction mixture was cooled, and formed solid was removed through filtration. The solid was recrystallized from acetone, and dried under vacuum, to thereby yield 518 mg of bis(p-tert-butylphenyl)iodonium camphorsulfonate (di(4-t-butylphenyl)iodonium camphorsulfonate) (yield: 83%).
 |
| | di(4-t-butylphenyl)iodonium camphorsulfonate Preparation Products And Raw materials |
|