| Company Name: |
Shaanxi Dideu Medichem Co. Ltd
|
| Tel: |
029-81103594 13389272314 |
| Email: |
1027@dideu.com |
| Products Intro: |
Product Name:Nopoxamine
CAS:7712-50-7
Purity:99%
|
|
| | myrtecaine Basic information |
| Product Name: | myrtecaine | | Synonyms: | myrtecaine;2-[2-(6,6-Dimethylnorpinan-2-en-2-yl)ethoxy]-N,N-diethylethanamine;Myrtecain;Nopoxamine;Ethanamine, 2-[2-(6,6-dimethylbicyclo[3.1.1]hept-2-en-2-yl)ethoxy]-N,N-diethyl-;MYRTECAINE LIQUID | | CAS: | 7712-50-7 | | MF: | C17H31NO | | MW: | 265.43 | | EINECS: | 231-735-7 | | Product Categories: | | | Mol File: | 7712-50-7.mol | 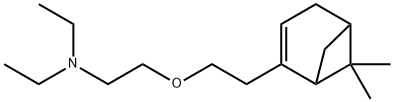 |
| | myrtecaine Chemical Properties |
| Melting point | <25 °C | | Boiling point | bp2-3 135-140° | | density | 0.930±0.06 g/cm3(Predicted) | | refractive index | nD20 1.477 | | pka | 9.77±0.25(Predicted) |
| | myrtecaine Usage And Synthesis |
| Originator | Myrtecaine,Chemical Land21 | | Manufacturing Process | 60 g (1.5 mol) of powdered sodium amide are put in suspension in 800 ml of
toluene. The mixture is heated to 60°C and 166 g of homomyrtenol (1 mol)
are added little by little. The reaction is continued for several hours until the
homomyrtenol is entirely converted into sodium derivative. It is allowed to
stand and the excess amide is filtered. The reaction is followed by titration on
a sample of the decanted liquid after having removed the ammonia.
Added to the solution of the sodium derivative of the terpenic alcohol (1 mol)
is a toluenic solution of 138 g (1.02 mol) diethylaminochloroethane in toluene.
This mixture is refluxed in a nitrogen atmosphere for 12 hours. A precipitate
of sodium chloride is formed which is dissolved in water. Two modes of
extraction of the base myrtecaine are possible:
A) The toluenic solution is extracted with two times 200 ml of concentrated
hydrochloric acid diluted to 20%. ln this way there is obtained an aqueous
solution of the hydrochloride, when the required amino base is salted out by
addition of potassium carbonate. The amino ether-oxide is finally rectified
under a vacuum. The fraction boiling between 135° and 140°C under 2 to 3
mm is collected; nD20 = 1.477.
B) The toluenic solution is dried on potassium carbonate and then rectified.
There is collected the toluene, then between 120° and 130°C under 2 mm the
homomyrtenol which has not reacted, and then between 130° and 145°C a
fraction which is again fractionated. The pure product is collected at 135°-
140°C/2-3mm Hg. | | Therapeutic Function | Local anesthetic, Spasmolytic |
| | myrtecaine Preparation Products And Raw materials |
|