- Wilforgine
-
- $0.00 / 5mg
-
2023-02-24
- CAS:37239-47-7
- Min. Order: 5mg
- Purity: ≥98%(HPLC)
- Supply Ability: 10 g
|
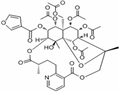
| Product Name: | WILFORGINE | | Synonyms: | (8alpha)-8-(Acetyloxy)-O2-deacetyl-8-deoxo-O2-(3-furanylcarbonyl)- evonimine Wilforgin;WILFORGINE;Aids088959;Aids-088959;3-Furancarboxylic acid (8R,9R,10R,11S,12R,13R,14R,15S,18S,21S,22S,23R)-10,13,22,23-tetrakis(acetyloxy)-12-[(acetyloxy)methyl]-7,8,9,10,12,13,14,15,17,18,19,20-dodecahydro-21-hydroxy-8,18,21-trimethyl-5,17-dioxo-8,11-epoxy-9,12-ethano-11,15-methano-5H,11H-[1,9]dioxacyclooctadecino[4,3-b]pyridin-14-yl ester;4-27-00-06847 (Beilstein Handbook Reference);3-Furancarboxylic acid (8R,9R,10R,11S,12R,13R,14R,15S,18S,21S,22S,23R)-10,13,22,23-tetrakis(acetyloxy)-;3-Furancarboxylic acid, 10,13,22,23-tetrakis(acetyloxy)-12-[(acetyloxy)methyl]-7,8,9,10,12,13,14,15,17,18,19,20-dodecahydro-21-hydroxy-8,18,21-trimethyl-5,17-dioxo-8,11-epoxy-9,12-ethano-11,15-methano-5H,11H-[1,9]dioxacyclooctadecino[4,3-b]pyridin-14-yl ester, (8R,9R,10R,11S,12S,13R,14R,15S,18S,21S,22S,23R)- | | CAS: | 37239-47-7 | | MF: | C41H47NO19 | | MW: | 857.81 | | EINECS: | | | Product Categories: | CR;reagent;standard substance | | Mol File: | 37239-47-7.mol |  |
| | WILFORGINE Chemical Properties |
| Melting point | 211℃ | | Boiling point | 859.9±65.0 °C(Predicted) | | density | 1.43 | | solubility | Soluble in methanol; | | pka | 11.59±0.70(Predicted) | | form | powder | | color | White | | Water Solubility | insoluble in water |
| | WILFORGINE Usage And Synthesis |
| Uses | Wilforgine can be used as lymphocyte immunosuppression, with anti-tumor and anti-inflammatory effects, and can be used for content determination/identification/pharmacological experiments, etc. | | Biological activity | Wilforgine is a bioactive sesquiterpene alkaloid in Tripterygium wilfordii Hook. F. Wilforgine can induce microstructural and ultrastructural changes in the muscles of Mythimna separata larvae, and the sites of action are proposed to be calcium receptors or channels in the muscular system. | | Description | Wilforgine is a alkaloid,it from Tripterygiurn wilfordii Hook forms colourless plates from
MeOH-Me2CO. It is also dextrorotatory having [α]25/sup>D + 25° (Me2CO)。 | | Uses | Wilforgine is a bioactive sesquiterpene alkaloid in Tripterygium wilfordii Hook. F. Wilforgine can induce microstructural and ultrastructural changes in the muscles of Mythimna separata larvae, and the sites of action are proposed to be calcium receptors or channels in the muscular system[1][2]. | | Definition | ChEBI: A dihydroagarofuran sesquiterpenoid and pyridine alkaloid with formula C41H47NO19 originally isolated from the roots of Tripterygium wilfordii. | | References | Beroza., J. Arner. Chern. Soc., 74, 1585 (1952)
Beroza., ibid, 75,44 (1953) |
| | WILFORGINE Preparation Products And Raw materials |
|