|
|
| | (S)-(+)-1,1'-Binaphthyl-2,2'-diyl hydrogenphosphate Basic information | | Reaction |
| Product Name: | (S)-(+)-1,1'-Binaphthyl-2,2'-diyl hydrogenphosphate | | Synonyms: | (S)-(+)-BNP ACID;(S)-(+)-BNPA;(S)-(+)-BPA;(S)-4-HYDROXY DINAPHTHO[2,1-D:1',2'-F][1,3,2]-DIOXAPHOPHEPIN-4-OXID;(S)-4-HYDROXYDINAPHTHO[2,1-D:1',2'-F][1,3,2]DIOXAPHOSPHEPIN-4-OXIDE;(S)-(+)-1,1'-BINAPHTHELENE-2,2'-DIYL-PHOSPHATE;(S)-(+)-1,1'-BINAPHTHYL-2,2'-DIYL HYDROGENPHOSPHATE;S-1,1'-BINAPHTHYL-2,2'-DIYL HYDROGENPHOSPHATE | | CAS: | 35193-64-7 | | MF: | C20H13O4P | | MW: | 348.29 | | EINECS: | 233-305-4 | | Product Categories: | Chiral Compound;Analytical Chemistry;e.e. / Absolute Configuration Determination (NMR);Enantiomer Excess & Absolute Configuration Determination;for Resolution of Bases;Optical Resolution;Synthetic Organic Chemistry;Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals;chiral;API intermediates;Chiral Compounds;Chiral Phosphine;bc0001 | | Mol File: | 35193-64-7.mol | 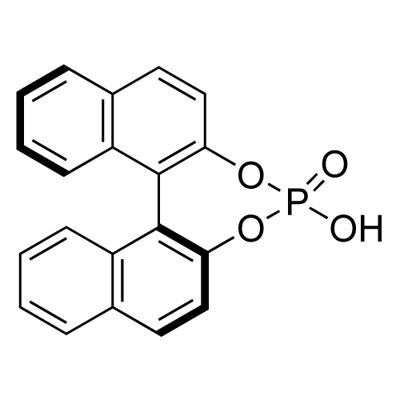 |
| | (S)-(+)-1,1'-Binaphthyl-2,2'-diyl hydrogenphosphate Chemical Properties |
| Melting point | ≥300 °C | | alpha | 607.5 º (c=1.4,MeOH) | | Boiling point | 619.5±38.0 °C(Predicted) | | density | 1.49±0.1 g/cm3(Predicted) | | refractive index | 595 ° (C=1, MeOH) | | storage temp. | Sealed in dry,Room Temperature | | solubility | Soluble in DMSO and methanol (hot). | | pka | 1.14±0.20(Predicted) | | form | Powder | | color | white | | optical activity | [α]22/D +595°, c = 1.35 in methanol | | BRN | 4787775 | | InChI | InChI=1S/C20H14O4P/c21-25(22)23-17-11-9-13-5-1-3-7-15(13)19(17)20-16-8-4-2-6-14(16)10-12-18(20)24-25/h1-12,19H,(H,21,22) | | InChIKey | LOHAVLUFTJNVNC-UHFFFAOYSA-N | | SMILES | P1(OC2=CC=C3C(=C2C2([H])=C4C(C=CC=C4)=CC=C2O1)C=CC=C3)(O)=O | | CAS DataBase Reference | 35193-64-7(CAS DataBase Reference) |
| | (S)-(+)-1,1'-Binaphthyl-2,2'-diyl hydrogenphosphate Usage And Synthesis |
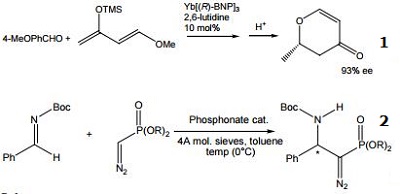
| Reaction |
- Asymmetric hetero Diels-Alder reaction catalyzed by chiral lanthanide(III) complex.
- Highly efficient Mannich reaction
- Acidic Resolving agent for certain amine/racemic mixtures.
| | Chemical Properties | white to light yellow crystal powde | | Uses | Used as a chiral Bronsted acid catalyst in the enantoselective Mannich reaction. | | Uses | The S enantiomer of binaphthol derivative as chiral quenching agent. | | Purification Methods | Recrystallise it from EtOH. Reflux for 3hours in N NaOH is required to hydrolyse the cyclic phosphate. [Jacques et al. Tetrahedron Lett 4617 1971, Arnold et al. Tetrahedron 24, 343 1983.] |
| | (S)-(+)-1,1'-Binaphthyl-2,2'-diyl hydrogenphosphate Preparation Products And Raw materials |
|