- Cyclen
-

- $0.00 / 25Kg/Drum
-
2025-04-03
- CAS:294-90-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100mt/year
- Cyclen
-

- $120.00 / 1kg
-
2025-04-02
- CAS:294-90-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Cyclen
-

- $0.00 / 25Kg/Bag
-
2025-03-21
- CAS:294-90-6
- Min. Order: 1Kg/Bag
- Purity: 99%
- Supply Ability: 5000kg/month
|
| | Cyclen Chemical Properties |
| Melting point | 110-113 °C (lit.) | | Boiling point | 292.61°C (rough estimate) | | density | 1.0415 (rough estimate) | | vapor pressure | 0.004Pa at 20℃ | | refractive index | 1.5872 (estimate) | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | Chloroform (Slightly), Methanol (Slightly) | | pka | 10.53±0.20(Predicted) | | form | Crystalline Powder | | color | Almost white to slightly yellow | | Water Solubility | almost transparency | | BRN | 606114 | | Stability: | hygroscopic | | InChIKey | QBPPRVHXOZRESW-UHFFFAOYSA-N | | LogP | -0.63 at 20℃ | | CAS DataBase Reference | 294-90-6(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/38-36/37/38 | | Safety Statements | 26-36 | | RIDADR | 1759 | | WGK Germany | 3 | | RTECS | XA5253000 | | F | 3-9-34 | | Hazard Note | Irritant | | HazardClass | 8 | | PackingGroup | II | | HS Code | 29339900 |
| | Cyclen Usage And Synthesis |
| Chemical Properties | almost white to slightly yellow crystalline powder, soluble in methanol, ethanol, dmso and other organic solvents, derived from stephania cepharantha. | | Uses | Cyclen is an azamocrocycle, which can be used in the development of fluorescent nanosensors for the detection of metal ions. | | Application | Cyclen (1,4,7,10-tetraazacyclododecane) is a macrocyclic aza analogue of the crown ether 12-crown-4. Cyclen compounds are capable of selectively binding cations and are used as a ligand with chemicals used in MRI contrast (as well ass other imaging) agents. | | Definition | ChEBI: 1,4,7,10-tetraazacyclododecane is an azacycloalkane that is cyclododecane in which the carbon atoms at positions 1, 4, 7 and 10 are replaced by nitrogen atoms. It is a saturated organic heteromonocyclic parent, a crown amine and an azacycloalkane. | | Synthesis Reference(s) | Journal of the American Chemical Society, 96, p. 2268, 1974 DOI: 10.1021/ja00814a056 | | General Description | Cyclen is a microcyclic tetramine that can be used as a ligand that forms a co-ordination linkage with the surface metal cations. It can be used as a synthetic precursor. It can be prepared by S-alkylation of dithiooxamide with an excess amount of bromoethane. | | Flammability and Explosibility | Not classified | | Synthesis | Some syntheses exploit the Thorpe-Ingold effect to facilitate ring-formation. Illustrative is the reaction of the deprotonated tosylamides with ditosylates:
TsN(CH2CH2NTsNa)2 + TsN(CH2CH2OTs)2 → (TsNCH2CH2)4
The resulting macrocycle can be deprotected with strong acid. Base gives the tetramine.
High dilution conditions result in a low reaction rate penalty and this disadvantage is removed in an alternative procedure starting from triethylenetetraamine and dithiooxamide to a bisamidine – also a bis(imidazoline) – followed by reduction and ring expansion with DIBAL.
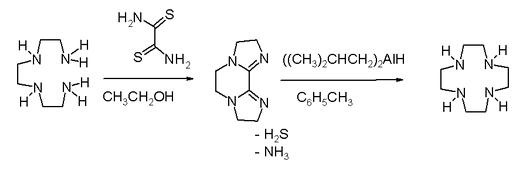
In one study cyclen is covalently bonded through a propylene molecular spacer to adenine and chelated with zinc diperchlorate. This complex is able to selectively bind uracil and uridine in a 1:2 ratio both through the adenine part and cyclen part of the molecule as evidenced by mass spectrometry.
wiki/Cyclen |
| | Cyclen Preparation Products And Raw materials |
| Raw materials | Hydrochloric acid-->Ethyl acetate-->Sulfuric acid-->Acetic acid-->Pyridine-->Sodium Methoxide-->Tosyl chloride-->Diethanolamine-->Diisopropyl ether-->Diethylenetriamine-->1,4,7,10-TETRA-P-TOSYL-1,4,7,10-TETRAAZACYCLODODECANE-->1-Bromo-2-iodoethane-->1,4,7,10-TETRAAZACYCLODODECANE TETRAHYDROCHLORIDE-->1-Bromo-2-chloroethane-->1,2-DIIODOETHANE-->1,2-Dibromoethane | | Preparation Products | TRI-TERT-BUTYL 1 4 7 10-TETRAAZACYCLODOD-->Ethylenediamine-->DOTA |
|