| | OXYCODONE Basic information |
| Product Name: | OXYCODONE | | Synonyms: | OXYCODONE;4,5-alpha-epoxy-14-hydroxy-3-methoxy-17-methyl-morphinan-6-on;4,5alpha-epoxy-14-hydroxy-3-methoxy-17-methyl-morphinan-6-on;4,5alpha-Epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one;eubine(france);Eucodalum;Morphinan-6-one, 4,5alpha-epoxy-14-hydroxy-3-methoxy-17-methyl-;Morphinan-6-one, 4,5-epoxy-14-hydroxy-3-methoxy-17-methyl-, (5alpha)- | | CAS: | 76-42-6 | | MF: | C18H21NO4 | | MW: | 315.36 | | EINECS: | 200-960-2 | | Product Categories: | API;phartmaceutical intermediate | | Mol File: | 76-42-6.mol | 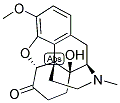 |
| | OXYCODONE Chemical Properties |
| | OXYCODONE Usage And Synthesis |
| Description | Oxycodone (Item No. 15465) is an analytical reference material categorized as an opioid. Oxycodone is regulated as a Schedule II compound in the United States. This product is intended for research and forensic applications. | | Originator | Oxyfast,Purdue Pharma, L.P. | | Uses | Analgesic (narcotic). | | Definition | oxycodone: An opioid, C18H21N2,similar in structure to codeine butwith a –OH group in codeine replacedby a carbonyl group. It is ananalgesic often used for the treatmentof chronic pain. It is also usedillegally and is a controlled substancein most countries. | | Brand name | Oxycontin (Purdue); Roxicodone (Roxane); Roxicodone (Xanodyne). | | Therapeutic Function | Narcotic analgesic | | Biological Functions | Oxycodone is nearly 10 times as strong as codeine,
with absorption equal to that of orally administered morphine.
Neither hydromorphone nor oxycodone is approved
for use in children, and hydromorphone is contraindicated
in obstetrical analgesia and in asthmatics. | | General Description | Oxycodone is synthesized from the natural opium alkaloidthebaine. Oxycodone is the 14 beta-hydroxyl version of hydrocodone.This additional functional group gives oxycodonegreater potency (1.5 times orally) than hydrocodone presumablyby increasing receptor affinity. The oral bioavailabilityof oxycodone is 65% to 87%. The metabolism of oxycodonefollows the similar pattern of opioid metabolism withN-demethylation, O-demethylation, and their glucuronides allidentified. Per the manufacturer, the analgesic effect of oxycodonecorrelates well with oxycodone plasma concentrations,not the minimal amount of oxymorphone formed, thusoxycodone is not assumed to be a prodrug. There are no largescalestudies of oxycodone used for analgesia in CYP2D6poor metabolizers that can confirm this.
Oxycodone is marketed in combination with acetaminophen(Percocet), aspirin (Percodan), and ibuprofen(Combunox). It has been available for over 50 years as animmediate-release tablet, and in 1995 an extended-releasetablet was approved by the FDA (OxyContin). OxyContin ismanufactured in eight strengths from 10 to 160 mg, and thehigh-dose preparations quickly became attractive to drugabusers. The extended-release tablets are crushed and theninjected or snorted to give an immediate high. The DrugAbuse Warning Network (DAWN) is a public health surveillancesystem that monitors drug-related emergency roomvisits and drug-related deaths. In 1995, they estimated that598,542 emergency room visits involved the nonmedical useof a pharmaceutical (e.g., antidepressant, anxiolytic, stimulant).Of these ER visits, 160,363 visits were attributed toopiates with an estimated 42,810 involving oxycodone or anoxycodone combination. Methadone and hydrocodone/combinationswere estimated to be similar to oxycodone. | | Pharmacology | Oxycodone is a potent semisynthetic opioid that has been in use for many
years. In addition to actions at the MOP receptor, it may also have analgesic
effects mediated via the KOP receptor, resulting in incomplete crosstolerance
with morphine. It has a good oral bioavailability, and its plasma
concentrations are more predictable than those of morphine after oral
administration. It is available in both long- and short-acting oral
preparations and, more recently, in a parenteral formulation. Oral oxycodone
is roughly 1.5 times more potent than oral morphine. | | Safety Profile | Poison by intraperitoneal route. Moderately toxic by subcutaneous route. When heated to decomposition it emits toxic fumes of NOx. |
| | OXYCODONE Preparation Products And Raw materials |
|