- Chromic acid USP/EP/BP
-

- $1.10 / 1g
-
2021-07-23
- CAS:7738-94-5
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
- Chromic acid
-

- $0.00 / 1KG
-
2020-05-04
- CAS:7738-94-5
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 600 Tons
- Chromic acid
-

- $1.00 / 1KG
-
2019-12-26
- CAS:7738-94-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 300kg
|
| | Chromic acid Basic information |
| Product Name: | Chromic acid | | Synonyms: | dihydroxy(diketo)chromium;CHROMIC ACID;Chromic acid USP/EP/BP;TIANFU-CHEM Chromic acid;Cromic acid | | CAS: | 7738-94-5 | | MF: | CrH2O4 | | MW: | 118.01 | | EINECS: | 231-801-5 | | Product Categories: | | | Mol File: | 7738-94-5.mol | 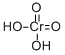 |
| | Chromic acid Chemical Properties |
| Melting point | 196°C | | Boiling point | 250 °C (decomposes) | | density | 2.290 | | solubility | Methanol (Slightly) | | form | Liquid | | color | Clear, orange | | PH | 3.03(1 mM solution);2.33(10 mM solution);2.06(100 mM solution) | | Odor | Odorless | | Water Solubility | HIGHLY Soluble | | EPA Substance Registry System | Chromic(VI) acid (7738-94-5) |
| | Chromic acid Usage And Synthesis |
| Description | Chromic acid, CrO3, is composed of dark, purplish-red, odorless crystals that are soluble in water. The specific gravity is 2.7, which is heavier than water. It is a powerful oxidizing agent and may explode on contact with organic materials. Chromic acid is a poison, corrosive to the skin, and has a TLV of 0.05 mg/m3 of air. Chromic acid is a known human carcinogen. The four-digit UN identification number is 1463. The NFPA 704 designation is health 3, flammability 0, and reactivity 1. The white section at the bottom of the 704 diamond has an “oxy” prefix, indicating that it is an oxidizer. | | Chemical Properties | Chromic acid is a dark purplish-red odorless flakes or crystalline powder | | Uses | Chemicals (chromates, oxidizing agents, catalysts), chromium-plating intermediate, medicine
(caustic), process engraving, anodizing, ceramic
glazes, colored glass, metal cleaning, inks, tanning,
paints, textile mordant, etchant for plastics. | | Definition | The name is in common use, although the true
chromic acid, H2CrO4, exists only in solution. | | Definition | chromic acid: A hypothetical acid,H2CrO4, known only in chromatesalts. | | General Description | Chromic acid is a dark purplish red solid, exists only in solution. The hydrate of chromiumoxide, it is used in electroplating baths. Chromic acid is soluble in water with the release of heat. The material itself is noncombustible but Chromic acid will accelerate the burning of combustible materials. Its solution is corrosive to metals and tissue. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | A very powerful oxidizing agent, confirmed human carcinogen. Upon contact with reducing reagents Chromic acid can cause a violent explosion, in contact with organic matter Chromic acid may cause a violent oxidation leading to ignition. Dangerously reactive with acetone, alcohols, alkali metals (sodium, potassium), ammonia, arsenic, dimethylformamide, hydrogen sulfide, phosphorus, peroxyformic acid, pyridine, selenium, sulfur, and many other chemicals [Sax, 9th ed., 1996, p. 852]. When mixed with sulfuric acid for glass cleaning operations, used solution in closed bottle may explode due to internal pressure of carbon dioxide arising from contamination by carbon compounds [Bryson, W. R., Chem. Brit., 1975, 11, p. 377]. | | Hazard | A human carcinogen. A poison. Corrosive
to skin. Powerful oxidizing agent, may explode on
contact with reducing agents, may ignite on contact with organic materials. Upper respiratory tract
irritant. | | Health Hazard | Very irritating to eyes and respiratory tract. Ingestion causes severe gastrointestinal symptoms. Contact with eyes or skin causes burns; prolonged contact produces dermatitis (``chrome sores''). | | Fire Hazard | Behavior in Fire: Containers may explode | | Safety Profile | Confirmed human
carcinogen. Poison by subcutaneous route.
Mutation data reported. A powerful
oxidzer. A powerful irritant of skin, eyes,
and mucous membranes. Can cause a
dermatitis, bronchoasthma, “chrome holes,”
damage to the eyes. Dangerously reactive.
Incompatible with acetic acid, acetic
anhydride, tetrahydronaphthalene, acetone,
alcohols, alkali metals, ammonia, arsenic,
bromine penta fluoride, butyric acid, n,ndimethylformamide, hydrogen sulfide,
peroxyformic acid, phosphorus, potassium
hexacyanoferrate, pyridme, selenium | | Potential Exposure | n chromium plating; medicine, ceramic glazers, and paints. | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24�48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray | | storage | Color Code—Yellow: Reactive Hazard; Store in alocation separate from other materials, especially flammables and combustibles. Color Code—Blue: HealthHazard/Poison: Store in a secure poison location. Prior toworking with chromic acid you should be trained on itsproper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from acetone, combustible, organic or other readily oxidizable material (such as paper, wood, sulfur, aluminum, and plastics).Sources of ignition, such as smoking and open flames, areprohibited where Chromic acid is used, handled, or storedin a manner that could create a potential fire or explosionhazard. A storage hazard; sealed containers may burst fromcarbon dioxide release. Store in tightly closed containers ina dry, cool, well-ventilated place with nonwood floors.Keep away from combustible materials; alcohols; and acetone. Where possible, automatically transfer chromic acidfrom drums or other storage containers to process containers. Containers may explode in fire. See OSHA Standard1910.104 and NFPA 43A Code for the Storage of Liquidand Solid Oxidizers for detailed handling and storage regulations. A regulated, marked area should be establishedwhere this chemical is handled, used, or stored in compliance with OSHA Standard 1910.1045 | | Shipping | UN1463 Chromium trioxide, anhydrous, Hazard Class: 5.1; Labels: 5.1-Oxidizer, 6.1-Poisonous materials, 8-Corrosive material. UN1755 (solution) Chromic acid, solid, Hazard class: 8; Labels: 8-Corrosive material. | | Incompatibilities | A strong oxidizer. Aqueous solution is strongly acidic. Reacts with acetic acid, acetic anhydride, acetone, anthracene, chromous sulfide; diethyl ether; dimethyl formamide; ethanol, hydrogen sulfide; methanol, naphthalene, camphor, glycerol, potassium ferricyanide, pyridine, turpentine, combustibles; organics, and other easily oxidized materials (such as paper, wood, sulfur, aluminum, and plastics). Attacks metals in presence of moisture | | Waste Disposal | Chemical reduction to chromium(III) can be followed by land fill disposal of the sludge. |
| | Chromic acid Preparation Products And Raw materials |
|