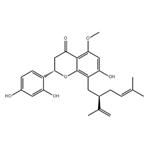
- Kurarinone
-

- $39.00 / 1mg
-
2024-11-18
- CAS:34981-26-5
- Min. Order:
- Purity: 99.72%
- Supply Ability: 10g
- KURARINONE
-
- $1.00 / 1KG
-
2024-08-11
- CAS:34981-26-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20T
- Kurarinone
-
- $0.00 / 5mg
-
2023-02-24
- CAS:34981-26-5
- Min. Order: 5mg
- Purity: ≥98%(HPLC)
- Supply Ability: 10 g
|
| | KURARINONE Basic information |
| Product Name: | KURARINONE | | Synonyms: | KURARINONE;Kurarinon;Marini;4H-1-Benzopyran-4-one,2-(2,4-dihydroxyphenyl)-2,3-dihydro-7-hydroxy-5-methoxy-8-[(2R)-5-methyl-2-(1-methylethenyl)-4-hexen-1-yl]-,(2S)-;2-(2,4-Dihydroxyphenyl)-2,3-dihydro-7-hydroxy-5-methoxy-8-[5-methyl-2-(1-methylethenyl)-4-hexenyl]-4H-1-benzopyran-4-one;Kurarinone >=98% (HPLC);2-(2,4-dihydroxyphenyl)-5,7-dihydroxy-8-(5-methyl-2-prop-1-en-2-ylhex-4-enyl)-2,3-dihydrochromen-4-one;(S)-2-(2,4-Dihydroxyphenyl)-7-hydroxy-5-methoxy-8-((R)-5-methyl-2-(prop-1-en-2-yl)hex-4-en-1-yl)chroman-4-one | | CAS: | 34981-26-5 | | MF: | C26H30O6 | | MW: | 438.51 | | EINECS: | 233-305-4 | | Product Categories: | | | Mol File: | 34981-26-5.mol | 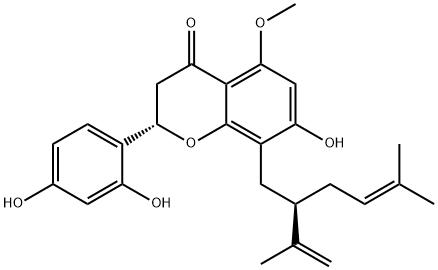 |
| | KURARINONE Chemical Properties |
| Melting point | 117-119℃ | | Boiling point | 651.4±55.0 °C(Predicted) | | density | 1?+-.0.06 g/cm3(Predicted) | | storage temp. | -20°C | | solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | | form | Powder | | pka | 7.49±0.40(Predicted) | | color | Light yellow to yellow | | biological source | (isolated from Caragana sinica) |
| | KURARINONE Usage And Synthesis |
| Uses | Kurarinone is an orally active flavonoid isolated from matrine that inhibits the pathogenesis of experimental autoimmune encephalomyelitis by inhibiting cell differentiation of Th1 and Th17. Kurarinone has antitumor and anti-inflammatory activity[1][2][3]. | | Definition | ChEBI: (2S)-(-)-kurarinone is a trihydroxyflavanone that is (2S)-flavanone substituted by hydroxy groups at positions 7, 2' and 4', a lavandulyl group at position 8 and a methoxy group at position 5. Isolated from the roots of Sophora flavescens, it exhibits cytotoxicity against human myeloid leukemia HL-60 cells. It has a role as a metabolite and an antineoplastic agent. It is a trihydroxyflavanone, a monomethoxyflavanone and a member of 4'-hydroxyflavanones. It is functionally related to a (2S)-flavanone. | | Biological Activity | Kurarinone, a flavonoid isolated from Sophora flavescens, inhibits the pathogenesis of experimental autoimmune encephalomyelitis by inhibiting the differentiation of Th1 and Th17 cells. | | Physiological effects | Kurarinon has an anti-endothelial cell proliferative activity, and it has a significant inhibitory effect on endothelial cell proliferation with a median inhibitory concentration (IC50) of 12ug/ml. | | in vivo | Kurarinone (100 mg/kg,口服,第 21-42 天) 在胶原诱导关节炎 (CIA) 小鼠模型中具有治疗作用[3]。
? Kurarinone (100 mg/kg,p.o.,from day 21-42) has a therapeutic effect in a mouse model of collagen-induced arthritis (CIA)[3].
| Animal Model: | CIA Mouse Model[3] | | Dosage: | 100 mg/kg | | Administration: | p.o. | | Result: | Reduced the levels of proinflammatory cytokines, TNF-α, IL-6, IFN-γ, and IL-17A.
Reduced STAT1 and STAT3 phosphorylation and the proportions of Th1 and Th17 cells in lymph nodes.
|
| | References | [1] Xie L, et al. The flavonoid kurarinone inhibits clinical progression of EAE through inhibiting Th1 and Th17 cell differentiation and proliferation. Int Immunopharmacol. 2018 Sep;62:227-236. DOI:10.1016/j.intimp.2018.06.022
[2] Nishikawa S, et al. Kurarinone from Sophora Flavescens Roots Triggers ATF4 Activation and Cytostatic Effects Through PERK Phosphorylation. Molecules. 2019 Aug 27;24(17):3110. DOI:10.3390/molecules24173110
[3] Tang KT, et al. Kurarinone Attenuates Collagen-Induced Arthritis in Mice by Inhibiting Th1/Th17 Cell Responses and Oxidative Stress. Int J Mol Sci. 2021 Apr 13;22(8):4002. DOI:10.3390/ijms22084002 |
| | KURARINONE Preparation Products And Raw materials |
|