- Vismodegib
-

- $0.00 / 25kg
-
2025-03-17
- CAS:879085-55-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS
- Vismodegib
-

- $0.00 / 1g
-
2025-01-13
- CAS:879085-55-9
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month
- Vismodegib.
-

- $0.00 / 1g
-
2025-01-13
- CAS:879085-55-9
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month
Related articles - What is Vismodegib
- Vismodegib (GDC-0449; Vismo) is a first-in-class, orally administered Hedgehog pathway inhibitor approved in 2012.
- Aug 1,2024
|
| | Vismodegib Basic information |
| Product Name: | Vismodegib | | Synonyms: | 2-Chloro-N-[4-chloro-3-(2-pyridinyl)phenyl]-4-(methylsulfonyl)benzamide;VisModegib (GDC-0449);BenzaMide, 2-chloro-N-[4-chloro-3-(2-pyridinyl)phenyl]-4-(Methylsulfonyl)-;GDC-0449,Vismodegib;2-Chloro-N-(4-chloro-3-(pyridin-2-yl)-phenyl)-4-(methylsulfonyl)benzamide;VisModegib Base;2-Chloro-N-[4-chloro-3-(2-pyridinyl)phenyl]-4-(methylsulfonyl)benzamide Vismodegib(GDC0449);Vismodegib 2-Chloro-N-[4-chloro-3-(2-pyridinyl)phenyl]-4-(methylsulfonyl)benzamide | | CAS: | 879085-55-9 | | MF: | C19H14Cl2N2O3S | | MW: | 421.3 | | EINECS: | 806-752-3 | | Product Categories: | Inhibitors;Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Inhibitor;API | | Mol File: | 879085-55-9.mol |  |
| | Vismodegib Chemical Properties |
| Melting point | 179-181°C | | Boiling point | 561.6±50.0 °C(Predicted) | | density | 1.440 | | storage temp. | -20° | | solubility | Soluble in DMSO (up to 200 mg/ml) or in Ethanol (up to 10 mg/ml with warming) | | form | White solid. | | pka | 10.72±0.70(Predicted) | | color | Off-white or beige | | Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. | | InChI | InChI=1S/C19H14Cl2N2O3S/c1-27(25,26)13-6-7-14(17(21)11-13)19(24)23-12-5-8-16(20)15(10-12)18-4-2-3-9-22-18/h2-11H,1H3,(H,23,24) | | InChIKey | BPQMGSKTAYIVFO-UHFFFAOYSA-N | | SMILES | C(NC1=CC=C(Cl)C(C2=NC=CC=C2)=C1)(=O)C1=CC=C(S(C)(=O)=O)C=C1Cl |
| | Vismodegib Usage And Synthesis |
| Description | In January 2012, the US FDA approved vismodegib (also referred to as
GDC-0449) for the treatment of adults with metastatic basal cell carcinoma
(BCC), with locally advanced BCC that has recurred following surgery or
who are not candidates for surgery or radiation. Vismodegib inhibits the Hh sig?naling pathway by functioning as an antagonist of SMO thereby inhibiting the activation of Hedgehog target genes, resulting in decreased downstream pro?duction of proliferation factors. The IC50 of vismodegib in a Hedgehog?responsive cell line derived from human embryonic palatal mesenchyme cells was 2.8 nM. In preclinical in vivostudies, vismodegib at 12.5 mg/kg (bid) caused complete regression of tumors in a Hh pathway dependent medulloblas?tomaallograft model generated from Ptch+/-mice. A synthesis of vismodegib starting from 2-chloro-5-nitro aniline and employing a Negishi coupling with 2-pyridyl zinc iodide as a key step has been reported. | | Chemical Properties | White Solid | | Originator | Curis/Genentech (United States) | | Uses | Vismodegib targets the Hedgehog (Hh) pathway. Inhibition of the Hh signaling may be effective in the treatment and prevention of many types of human cancers. Potent Hedgehog inhibitor. | | Uses |
Vismodegib (GDC-0449) is a potent, novel and specific hedgehog inhibitor with IC50 of 3 nM and also inhibits P-gp with IC50 of 3.0 μM. Palliative Use of Vismodegib Nonmelanoma skin cancer (NMSCa) is often seen in elderly patients with multiple comorbidities and functional impairments resulting in treatment challenges. In 2012, the Food and Drug Administration (FDA) approved vismodegib for treatment of metastatic basal cell carcinoma (BCC), locally advanced BCC recurrent following surgery, and for patients who are not surgical or radiation candidates.
| | Definition |
ChEBI:Vismodegib is a benzamide obtained by formal condensation between the carboxy group of 2-chloro-4-(methylsulfonyl)benzoic acid and the anilino group of 4-chloro-3-(pyridin-2-yl)aniline. Used for the treatment metastatic basal cell carcinoma. It has a role as an antineoplastic agent, a SMO receptor antagonist, a Hedgehog signaling pathway inhibitor and a teratogenic agent. It is a sulfone, a member of benzamides, a member of pyridines and a member of monochlorobenzenes.
| | Brand name | Erivedge | | Clinical Use | Antineoplastic agent:
Treatment of basal cell carcinoma which is inappropriate for surgery or radiotherapy | | Synthesis | The synthesis began with selective iodination of commercial carboxylic acid 179, affording
trisubstituted arene 180 in 73% yield. A Curtius reaction then converted 180 to carbamate 181 in 84%
52
yield, and this was followed by a palladium(0)-catalyzed borylation of 181 which furnished Suzuki
coupling partner 182 in 91% yield. Pinacol borane 182 was exposed to commercial 2-bromopyridine
under conventional cross-coupling conditions to furnish biaryl 183, which underwent Boc-deprotection
in quantitative conversion to generate 184. Amide bond formation with acid chloride 185 (readily
available from the corresponding commercial acid) produced vismodegib (XXVIII) in 99% yield. 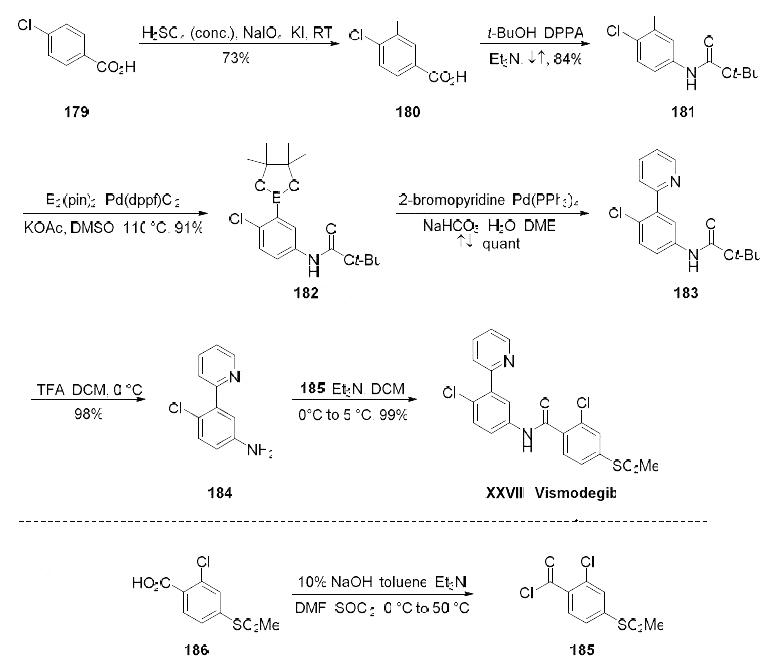
| | target | Hedgehog | | Drug interactions | Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly reduced by
rifampicin - avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration possibly reduced by
carbamazepine, fosphenytoin and phenytoin - avoid.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis. | | Metabolism | Vismodegib is hepatically metabolised by CYP2C9 and
CYP3A4, however more than 98
% of total systemic
vismodegib is not metabolised. Metabolic pathways
of vismodegib include oxidation, glucuronidation, and
pyridine ring cleavage. The two most abundant oxidative
metabolites recovered in faeces are produced in vitro by
recombinant CYP2C9 and CYP3A4/5.
Vismodegib is slowly eliminated by a combination of
metabolism and excretion of parent drug, the majority
is recovered in the faeces (82
%). Vismodegib and its
metabolites are eliminated mainly by the hepatic route. | | storage | Store at -20°C | | References | 1) Rominger et al. (2009), Evidence for allosteric interactions of antagonist binding to the smoothened receptor; J. Pharmacol. Exp. Therap., 329 995
2) Tian et al. (2012), The hedgehog pathway inhibitor GDC-0449 alters intracellular Ca2+ homeostasis and inhibits cell growth in cisplatin-resistant lung cancer cells; Anticancer Res., 32 89
3) Zhang et al. (2009), Hedgehog pathway inhibitor HhAntag691 is a potent inhibitor of ABCG2/BCRP and ABCB1/Pgp; Neoplasia, 11 96
4) Cirrone and Harris (2012), Vismodegib anf the hedgehog pathway: a new treatment for basal cell carcinoma; Clin. Ther., 34 2039
5) Wu et al. (2017), Smoothened antagonist GDC-0449 (Vismodegib) inhibits proliferation and triggers apoptosis in colon cancer cell lines; Exp. Ther. Med., 13 2529
6)Singh et al. (2011) Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms; PLoS One 6?e27306
7) Rudin et al. (2009) Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449; N. Engl. J. Med., 361?1173
8) Von Hoff et al. (2009) Inhibition of the hedgehog pathway in advanced basal-cell carcinoma; N. Engl. J. Med., 361?1164 |
| | Vismodegib Preparation Products And Raw materials |
|