- RegorafenibHydrate
-

- $0.00 / 1kg
-
2025-03-07
- CAS:1019206-88-2
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20tons
- Regorafenib Hydrate
-

- $0.00 / 1g
-
2025-01-13
- CAS:1019206-88-2
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month
|
| | Regorafenib monohydrate Basic information |
| Product Name: | Regorafenib monohydrate | | Synonyms: | 4-[4-[[[[4-Chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]-3-fluorophenoxy]-N-methyl-2-pyridinecarboxamide hydrate;Regorafenib monohydrate;Regorafenib (BAY 73-4506)Monohydrate;Regorafenib hydrate;BAY 73-4506 Monohydrate;Regorafenib monohydrate 4-[4-[[[[4-Chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]-3-fluorophenoxy]-N-methyl-2-pyridinecarboxamide hydrate;4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methyl-2-pyridinecarboxamide hydrate (1:1);4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide,hydrate | | CAS: | 1019206-88-2 | | MF: | C21H17ClF4N4O4 | | MW: | 500.83 | | EINECS: | 1308068-626-2 | | Product Categories: | API | | Mol File: | 1019206-88-2.mol | 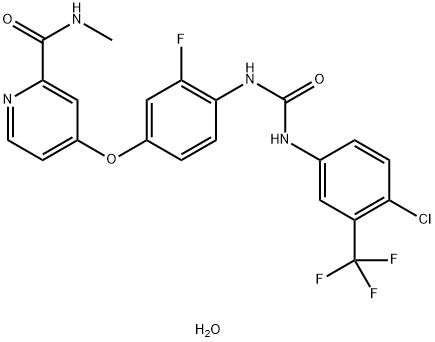 |
| | Regorafenib monohydrate Chemical Properties |
| storage temp. | 2-8°C | | solubility | ≥25.05 mg/mL in DMSO; insoluble in H2O | | form | solid | | color | Light yellow to orange | | InChIKey | ZOPOQLDXFHBOIH-UHFFFAOYSA-N | | SMILES | O(C1C=CC(NC(=O)NC2C=CC(Cl)=C(C(F)(F)F)C=2)=C(F)C=1)C1=CC=NC(C(=O)NC)=C1.O |
| | Regorafenib monohydrate Usage And Synthesis |
| Description | Regorafenib monohydrate (BAY 73-4506) is an orally active and potent multi-targeted receptor tyrosine kinase inhibitor with potent anti-tumour and anti-angiogenic activity. It is approved for the treatment of metastatic colorectal cancer, advanced gastrointestinal mesenchymal stromal tumours and hepatocellular carcinoma. | | Uses | 4-[4-[[4-Chloro-3-(trifluoromethyl)phenyl]carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide Hydrate can be used as an anti-tumor drug. | | Definition | ChEBI: Regorafenib hydrate is a hydrate that is the monohydrate form of anhydrous regorafenib. Used for for the treatment of metastatic colorectal cancer in patients who have previously received chemotherapy, anti-EGFR or anti-VEGF therapy. It has a role as an antineoplastic agent, a tyrosine kinase inhibitor and a hepatotoxic agent. It contains a regorafenib. | | Biological Activity | regorafenib monohydrate is a multitargetedinhibitor of tyrosine kinase with ic50 values of 13nm, 4.2nm, 46nm, 2.5nm, 28nm, 19nm, 202nm, 22nm, 7nm, 1.5nm and 311nm, respectively for vegfr-1, mvegfr-2, mvegfr-3, raf-1, braf wt, brafv600e, fgfr-1, pdgfr-β, c-kit, retand tie2 [1].regorafenib is a multikinase inhibitor of both intracellular and membrane-bound rtks. it shows potent inhibition of angiogenic and stromal rtks like vegf receptors-1-3, pdgfr-β and fgf receptor-1 with ic50 values ranging from4.2 to 311nm in biochemical assays. it also inhibits oncogenic rtks, such as ret and c-kit, with ic50 values ranging from 1.5 to 28nm in cellular assays [1].regorafenib is reported to have anti-tumor efficacy to various tumors including breast, pancreas, thyroid, melanoma, gist, and crc with a mean ic50 value less than 1μm. these inhibition effects of tumor growth are also found in mouse xenograft models after the treatment of regorafenib at dose ranging from 10 to 100 mg/kg [1]. | | Mechanism of action | The mechanism of anticancer action of Regorafenib monohydrate is similar to that of Regorafenib, an oral multikinase inhibitor that blocks tyrosine kinases that are active in angiogenesis, cancer development and growth, and maintenance of the tumour microenvironment. It is superior to sorafenib in blocking both vascular endothelial growth factor receptor and TIE2, a molecule with an important role in angiogenesis. | | Clinical Use | Regorafenib was approved by the U.S. Food and Drug Administration (FDA) in September 2012 for
the treatment of metastatic colorectal cancer in patients who have previously undergone
fluoropyrimidine-, oxaliplatin-, and irinotecan-based therapies. The FDA expanded the approved use
of the drug to include patients with advanced gastrointestinal stromal tumors (GIST) that cannot be
surgically removed and no longer respond to imatinib and sunitinib, two other drugs approved for
treatment of GIST. Regorafenib, marketed under the trade name Stivarga®, was discovered and
developed by Bayer Pharmaceuticals and marketed jointly with Onyx Pharmaceuticals. The active
metabolites of the drug inhibit multiple targets within a variety of kinase families including those in the RET, VEGF, FGFR, PTK, and Abl pathways. | | Side effects | Common side effects of regorafenib monohydrate include hand-foot skin reaction, diarrhea, nausea, vomiting, decreased appetite, fatigue, hypertension, and fever. | | Synthesis | Among several published synthesis, the most likely process scale synthesis will be highlighted
from the two published syntheses, and this is described in the scheme. Commercially available
picolinic acid (148) was heated with thionyl chloride to provide the crude intermediate 4-chloro-2-
pyridyl acid chloride which was subsequently reacted with aqueous methyl amine in toluene to give 4-
chloro-2-methylcarboxamide as its hydrochloride salt 149 in quantitative yield after treatment with
acetyl chloride in toluene and ethanol. The hydrochloride salt was free based with sodium hydroxide
and then immediately reacted with imine 150 (formed upon exposure to 4-amino-3-fluorophenol (153)
in refluxing 3-methyl 2-butanone) in base to provide diaryl ether 151 in 84% yield. Reaction of amine
151 with the commercially available isocyanate 152 ultimately delivered regorafenib hydrate (XXIII) in
83% yield. 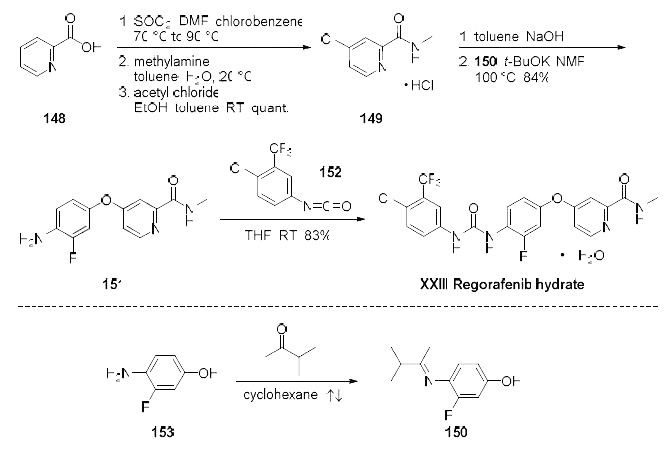
| | references | [1] crona dj, keisler md, walko cm.regorafenib: a novel multitargeted tyrosine kinase inhibitor for colorectal cancer and gastrointestinal stromal tumors.ann pharmacother. 2013 dec;47(12):1685-96. |
| | Regorafenib monohydrate Preparation Products And Raw materials |
|