|
|
| | (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester Basic information |
| Product Name: | (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester | | Synonyms: | (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester;1-Pyrrolidinecarboxylic acid, 4-oxo-2-(3-thiazolidinylcarbonyl)-, 1,1-diMethylethyl ester, (2S)-;(2S)-4-oxo-2-(1,3-thiazolidin-3-ylcarbonyl)pyrrolidine-1-carboxylate;(S)-tert-Butyl 4-oxo-2-(thiazolidine-3-carbonyl)pyrrolidine-1-carboxylate;(2S)-tert-butyl 4-hydroxy-2-(thiazolidine-3-carbonyl)pyrrolidine-1-carboxylate;Teneligptin interMediate B;tert-butyl 4-oxo-2-(thiazolidine-3-carbonyl)pyrrolidine-1-carboxylate;(2S)-4-Oxo-2-(thiazolidine-3-carbonyl)-pyrrolidine-1-carboxylic acid t-butyl ester | | CAS: | 401564-36-1 | | MF: | C13H20N2O4S | | MW: | 300.37 | | EINECS: | 813-401-8 | | Product Categories: | | | Mol File: | 401564-36-1.mol | 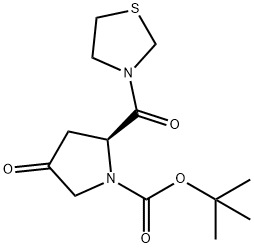 |
| | (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester Chemical Properties |
| Boiling point | 492℃ | | density | 1.305 | | Fp | 251℃ | | storage temp. | 2-8°C | | solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | | pka | -1.22±0.20(Predicted) | | form | Solid | | color | Pale Yellow to Light Yellow | | InChI | InChI=1S/C13H20N2O4S/c1-13(2,3)19-12(18)15-7-9(16)6-10(15)11(17)14-4-5-20-8-14/h10H,4-8H2,1-3H3/t10-/m0/s1 | | InChIKey | ULXKZRPRLJGLDM-JTQLQIEISA-N | | SMILES | N1(C(OC(C)(C)C)=O)CC(=O)C[C@H]1C(N1CCSC1)=O |
| | (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester Usage And Synthesis |
| Uses | (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic Acid 1,1-Dimethylethyl Ester is an intermediate used to prepare dipeptidyl peptidase IV (DPP-IV) inhibitors. | | Synthesis | To (2S)-1-tertbutyloxycarbonyl-4-oxa-tetramethyleneimine-2-carboxylic
acid (60.0kg), thiazolidine (30.3kg) and the solution
of N-diisopropylethylamine (118kg) in ethyl acetate (595kg), adds the
solution of 28w% propyl phosphonous acid anhydride (cyclic trimer)
in ethyl acetate (446kg) at 2 ℃-7 ℃, and reaction mixture is stirred 2
hours at 2 ℃-4 ℃.To this reaction mixture, add 15w% aqueous citric acid
solution (600kg) for distributing, and ethyl acetate for water layer
(271kg) is extracted.
The ethyl acetate layer obtaining is mixed, and use
successively 10w% ammonium dibasic phosphate aqueous solution (600kg)
and water (300kg) washing. Ethyl acetate layer is concentrated into
residual quantity 300L, normal heptane (739kg), 23 ℃ of-25 ℃ of
interpolations, and is stirred this mixture 1 hour at 23 ℃-25 ℃, and
stir 2 hours at 1 ℃-5 ℃.
The crystal of precipitation is collected by
filtration, is used normal heptane (164kg) washing drying under reduced
pressure to produce (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester (67.8kg, yield 86%).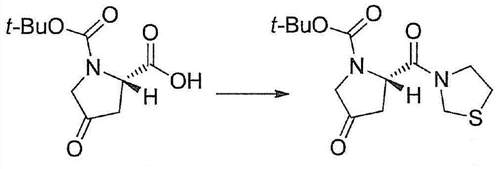
|
| | (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester Preparation Products And Raw materials |
|