Cariprazine hydrochloride manufacturers
|
| | Cariprazine hydrochloride Basic information |
| Product Name: | Cariprazine hydrochloride | | Synonyms: | Cariprazine (hydrochloride);RGH 188 hydrochloride;RGH188 hydrochloride;RGH-188 hydrochloride;Cariprazine(RGH 188);3-[4-[2-[4-(2,3-dichlorophenyl)piperazin-1-yl]ethyl]cyclohexyl]-1,1-dimethylurea,hydrochloride;3-[trans-4-[2-[4-(2,3-Dichlorophenyl)-1-piperazinyl]ethyl]cyclohexyl]-1,1-dimethylurea Hydrochloride;3-((1r,4r)-4-(2-(4-(2,3-Dichlorophenyl)piperazin-1-yl)ethyl)cyclohexyl)-1,1-dimethylureahydrochloride | | CAS: | 1083076-69-0 | | MF: | C21H33Cl3N4O | | MW: | 463.87 | | EINECS: | 200-077-2 | | Product Categories: | | | Mol File: | 1083076-69-0.mol | 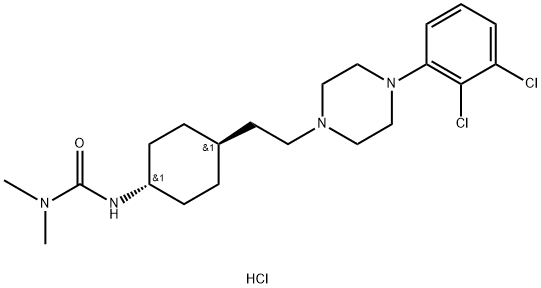 |
| | Cariprazine hydrochloride Chemical Properties |
| storage temp. | Store at -20°C | | solubility | DMSO : 6.67 mg/mL (14.38 mM; Need ultrasonic) | | form | Powder | | color | White to off-white | | InChI | InChI=1/C21H32Cl2N4O.ClH/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23;/h3-5,16-17H,6-15H2,1-2H3,(H,24,28);1H/t16-,17-; | | InChIKey | GPPJWWMREQHLQT-BHQIMSFRNA-N | | SMILES | ClC1C(=CC=CC=1N1CCN(CC[C@@H]2CC[C@@H](NC(=O)N(C)C)CC2)CC1)Cl.Cl |&1:13,16,r| |
| | Cariprazine hydrochloride Usage And Synthesis |
| Description | Cariprazine
hydrochloride (IX) is an oral, brain-penetrant, atypical
antipsychotic developed by the Hungarian pharmaceutical
firm Gedeon Richter. It was approved by the FDA in September 2015 for treatment of schizophrenia and for the
acute treatment of manic or mixed episodes of bipolar I
disorder. While the precise mechanism of action of
cariprazine is unknown, its antipsychotic and procognitive
effects may be mediated through partial agonism at dopamine
D2/D3 and serotonin 5-HT1A receptors as well as antagonism
at serotonin 5-HT2A receptors. Unlike many antipsychotics,
cariprazine displays particular selectivity for the D3 receptor
(D3, Ki = 0.085 nM; D2L, Ki = 0.49 nM; D2S, Ki = 0.69 nM).
Cariprazine is extensively metabolized by CYP3A4 and, to a
lesser extent, CYP2D6; desmethyl and didesmethyl cariprazine,
the primary metabolites, are pharmacologically equipotent to
the parent drug. In clinical trials, cariprazine demonstrated
improvement compared to placebo as measured by
Young Mania Rating Scale (YMRS) total scores in patients with
bipolar mania and by Positive and Negative Syndrome Scale
(PANSS) total scores in patients with schizophrenia. Forest
Laboratories (now Allergan) has exclusive rights to cariprazine
in the U.S. and Canada, while Mitsubishi Pharma Corporation
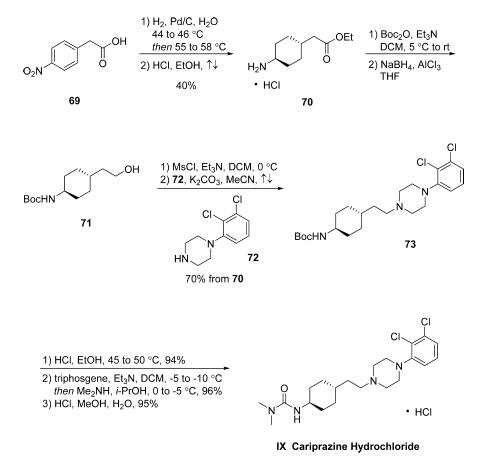
has exclusive rights to the sale of the drug in Japan and Asia. | | Uses | Cariprazine is used to treat schizophrenia (a mental illness that causes disturbed or unusual thinking, loss of interest in life, and strong or inappropriate emotions). Cariprazine is also used to treat episodes of depression in people with bipolar I disorder (manic depressive disorder; a disease that causes episodes of mania, episodes of depression and other abnormal moods). It is also used as a short term treatment for episodes of mania or mixed episodes (symptoms of mania and depression that happen together) in people with bipolar I disorder. Cariprazine is in a class of medications called atypical antipsychotics. It works by changing the activity of certain natural substances in the brain. | | Definition | ChEBI: Cariprazine hydrochloride is a hydrochloride obtained by combining cariprazine with one molar equivalent of hydrochloric acid. Used for treatment of schizophrenia and bipolar disorder. It has a role as a second generation antipsychotic, a dopamine agonist and a serotonergic antagonist. It contains a cariprazine(1+). | | Side effects | Common side effects of Cariprazine hydrochloride include: extreme tiredness, restlessness and anxiety, difficulty sleeping, dizziness, feeling unsteady or difficulty in maintaining balance; increased appetite or weight gain; constipation, dyspepsia, nausea, increased salivation or drooling. More serious side effects may include: uncontrollable unusual movements of the body or face; slow or shuffling walking; loss of mobility, fever, sweating, confusion, shortness of breath, fast or irregular heartbeat; and severe muscle stiffness; muscle weakness or soreness; blank facial expression; difficulty swallowing or breathing; tightness in the throat; tongue spitting, rash, itching, measles; face, throat, tongue, lips, or eyes are swollen; dark or cola-coloured urine; swelling of the legs and feet; and decreased urination. | | Synthesis | While the synthesis of cariprazine hydrochloride has been
reported in a number of patents as well as its discovery
synthesis in the publicly disclosed literature, the process route
has not yet been disseminated. Starting with the reduction of commercial 2-(4-
nitrophenyl)acetic acid (69) via hydrogenation in water in
the presence of Pd/C, this reaction proceeds a one-pot,
stepwise reduction of the nitro group. A separate reduction
event converting the phenyl ring to the corresponding
cyclohexane provides 4-aminocyclohexylacetic acid with 60-70% selectivity for the desired trans isomer. Following filtration
and distillation, the crude aqueous solution was treated with
HCl in refluxing ethanol to generate the corresponding ethyl
ester 70. Crystallization from acetonitrile gave the HCl salt in
high purity and 40% yield over two steps (a reaction sequence
that was reported on 200 kg scale). Amine 70 was transformed
into intermediate 73 via Boc protection followed by ester
reduction to the primary alcohol 71, which was obtained as a
solution in toluene following extraction. Next, mesylation of the
alcohol followed by alkylation with commercially available
piperazine 72 provided piperazinyl cyclohexane 73 in 70% over
the four-step sequence. The carbamate protecting group within
73 was removed via acidic ethanolysis, and the resulting
product was treated with triphosgene and dimethylamine to
generate cariprazine as the freebase. Salt formation by means of
methanolic HCl ultimately furnished cariprazine hydrochloride
IX in 85% yield from 73.
|
| | Cariprazine hydrochloride Preparation Products And Raw materials |
|