- Trimellitic Anhydride
-

- $99.00/ kg
-
2024-11-03
- CAS:552-30-7
- Min. Order: 0.0010000000474974513kg
- Purity: 99%
- Supply Ability: 5000
- Trimellitic Anhydride
-

- $0.00 / 25KG
-
2024-11-02
- CAS:552-30-7
- Min. Order: 25KG
- Purity: 98%min
- Supply Ability: 30tons/month
- Trimellitic Anhydride
-

- $99.00 / 1kg
-
2024-11-01
- CAS:552-30-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
|
| | Trimellitic Anhydride Basic information |
| Product Name: | Trimellitic Anhydride | | Synonyms: | 1,2,4-Benzenetricarboxylic acid anhydride-1,2;1,2,4-Benzenetricarboxylic acid, cyclic 1,2-anhydride;1,2,4-benzenetricarboxylicacid,cyclic1,2-anhydride;1,2,4-benzenetricarboxylicacidanhydride;1,3-dihydro-1,3-dixoxo-5-isobenzofurancarboxylic acid;benzene-1,2,4-tricarboxylic acid 1,2-anhydride trimellitic anhydride;BENZENE-1,3,4-TRICARBOXYLICANHYDRIDE;1,3-dioxo-5-phthalanacarboxylic acid | | CAS: | 552-30-7 | | MF: | C9H4O5 | | MW: | 192.13 | | EINECS: | 209-008-0 | | Product Categories: | fine chemicals;pharmaceutical raw material;Pyridines;Fluorenes, etc. (reagent for high-performance polymer research);Functional Materials;Reagent for High-Performance Polymer Research;Industrial/Fine Chemicals;addictives;bc0001;552-30-7 | | Mol File: | 552-30-7.mol | 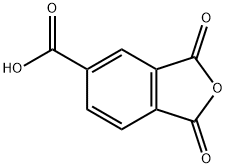 |
| | Trimellitic Anhydride Chemical Properties |
| Melting point | 163-166 °C (lit.) | | Boiling point | 390 °C | | density | 1.54 | | vapor density | 6.6 (vs air) | | vapor pressure | <0.01 mm Hg ( 20 °C) | | refractive index | 1.4717 (estimate) | | Fp | 227 °C | | storage temp. | Store below +30°C. | | solubility | 24.4g/l (decomposition) | | form | Flakes | | pka | 3.11±0.20(Predicted) | | color | White to off-white | | PH | 2 (21g/l, H2O, 20℃) | | explosive limit | 1-7%(V) | | Water Solubility | DECOMPOSES | | Sensitive | Moisture Sensitive | | Merck | 14,9703 | | BRN | 9394 | | Exposure limits | ACGIH: TWA 0.0005 mg/m3; STEL 0.002 mg/m3 (Skin)
NIOSH: TWA 0.005 ppm(0.04 mg/m3) | | Stability: | Stable. Moisture sensitive. | | InChIKey | SRPWOOOHEPICQU-UHFFFAOYSA-N | | LogP | 0.06-0.54 at 20℃ | | CAS DataBase Reference | 552-30-7(CAS DataBase Reference) | | NIST Chemistry Reference | Trimellitic anhydride(552-30-7) | | EPA Substance Registry System | Trimellitic anhydride (552-30-7) |
| Hazard Codes | Xn | | Risk Statements | 37-41-42/43 | | Safety Statements | 22-26-36/37/39 | | OEB | D | | OEL | TWA: 0.005 ppm (0.04 mg/m3) Should be handled in the workplace as an extremely toxic substance. | | WGK Germany | 1 | | RTECS | DC2050000 | | F | 10-21 | | TSCA | Yes | | HS Code | 29173980 | | Hazardous Substances Data | 552-30-7(Hazardous Substances Data) | | Toxicity | LD50 orally in Rabbit: > 2730 mg/kg LD50 dermal Rabbit 23000 mg/kg |
| | Trimellitic Anhydride Usage And Synthesis |
| Chemical Properties | Trimellitic anhydride is a white powder or flakes. It is the anhydride of trimellitic acid (1,2,4-benzenetricarboxylic acid). It is soluble in water, ethanol, acetone and other general organic solvents. | | Uses | Trimellitic Anhydride is mainly used as raw material for polyester resin,polyimide resin,plasticizer TOTM ,and also be used for producing heatproof & insulating,adhesive,surfactant, paint,synthesize dye materials etc.
Trimellitic anhydride is used as an embossing agent for vinyl flooring and as a curing agent for epoxy resins. It is also used as an intermediate for the synthesis of surface coatings chemicals, adhesives, polymers, dyes printing inks, pharmaceuticals . | | Uses | Trimellitic anhydride is mainly applied to produce good heat-proof, weather-proof and solvent-proof trimellitate plasticizers, among which the most popular product is tri(2-ethylhexyl)trimellitate. It is also used to synthesize polyester resins.TMA can also be applied to curing agent of epoxy resins. | | Definition | ChEBI: Trimellitic anhydride is a 2-benzofuran compound having oxo groups at the 1- and 3-positions and a carboxy substituent at the 5-position; the cyclic anhydride formed from the carboxy groups at the 1- and 2-positions of trimellitic acid. It has a role as an epitope, an allergen and a hapten. It is a cyclic dicarboxylic anhydride, a dioxo monocarboxylic acid and a member of 2-benzofurans. It is functionally related to a phthalic anhydride and a trimellitic acid. | | General Description | 1,2,4-Benzenetricarboxylic anhydride(TA) is a hydrophilic monomer with multipurpose usage; as a curing agent for epoxy based resins and as a plasticizer for polyvinyl chloride(PVC). | | Air & Water Reactions | Sensitive to moisture. Hydrolyzes slowly. Solutions in water or alcohol may be unstable. Insoluble in water. | | Reactivity Profile | 1,2,4-Benzenetricarboxylic anhydride reacts exothermically with water. This reaction is expected to be slow, but can become vigorous if local heating accelerates it. Reaction with water is accelerated by acids. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases. Incompatible with strong oxidizing agents, strong acids or strong bases. . | | Hazard | Toxic by inhalation. Respiratory sensitization. | | Health Hazard | Trimellitic anhydride (TMAN)
causes both respiratory irritation and immunologic
respiratory disease. | | Safety Profile | Moderately toxic by
ingestion. Has caused pulmonary edema
from inhalation. Irritant to lungs and air
passages. May be a powerful allergen.
Typical attack consists of breathlessness,
wheezing, cough, running nose,
immunologcal sensitization, and asthma
symptoms. When heated to decomposition
it emits acrid smoke and irritating fumes.
See also ANHYDRIDES. | | Synthesis | 3-Xylene is carbonylated with carbon monoxide in the presence of boron trifluoride and hydrogen fluoride to form 2,4-dimethylbenzaldehyde. 2,4-Dimethylbenzaldehyde is decomplexed from the acids, purified, and oxidized to trimellitic acid. Trimellitic acid is subjected to normal dehydration and purification steps to obtain high quality trimellitic anhydride. | | Potential Exposure | TMA is used to produce trimellitate
plasticizers, poly (amide-imide) polymers; in paints, enamels,
and coatings; polymers, polyesters; as a curing agent for
epoxy and other resins; in vinyl plasticizers; agricultural chemicals;
dyes and pigments; pharmaceuticals, surface active
agents; modifiers, intermediates, and specialty chemicals. | | Carcinogenicity | There are no reports of carcinogenicity
associated with TMAN exposure. It was not
mutagenic in bacterial assays with or without
metabolic activation. No teratogenic effects
or developmental toxicity was seen in rats
or guinea pigs exposed to 500mg/m3 for
6 hours/day during their period of major
organogenesis. | | Incompatibilities | Dust can cause an explosion.
Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep away
from alkaline materials, strong bases, strong acids, oxoacids,
epoxides. Reacts slowly with water, forming trimellitic
acid. Compounds of the carboxyl group react with all
bases, both inorganic and organic (i.e., amines) releasing
substantial heat, water and a salt that may be harmful. | | Waste Disposal | Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator
equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed. |
| | Trimellitic Anhydride Preparation Products And Raw materials |
| Raw materials | Polyvinyl chloride-->Triethylene glycol dimethacrylate-->Cobalt acetate-->1,2,4-Trimethylbenzene--> Manganese(II) acetate-->1,1,2,2-Tetrabromoethane-->POLY(1-GLYCEROL METHACRYLATE)-->ALKYD RESIN-->Polyurethane-polyvinyl chloride imitation leather-->DIOCTYL PHTHALATE (DOP)-->Curing agent for epoxy resin-->POLYIMIDE RESIN | | Preparation Products | Triethylene glycol dimethacrylate-->Rhodamine 123-->Triisooctyl phosphite-->5-Isobenzofurancarboxylic acid, 1,3-dihydro-1,3-dioxo-, 5,5'-[(1-methylethylidene)di-4,1-phenylene] ester |
|