|
|
| | CHLORODIMETHYLVINYLSILANE Basic information |
| | CHLORODIMETHYLVINYLSILANE Chemical Properties |
| Boiling point | 82-83 °C(lit.) | | density | 0.874 g/mL at 25 °C(lit.) | | vapor pressure | 95hPa at 20℃ | | refractive index | n20/D 1.414(lit.) | | Fp | 23 °F | | storage temp. | Inert atmosphere,2-8°C | | solubility | most aprotic organic solvents, but CH2Cl2 is most
commonly used; reacts with H2O and other protic solvents. | | form | clear liquid | | color | Colorless to Light yellow to Light orange | | Specific Gravity | 0.884 | | Water Solubility | Reacts with water. | | Sensitive | Moisture Sensitive | | Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents | | BRN | 1737688 | | InChIKey | XSDCTSITJJJDPY-UHFFFAOYSA-N | | CAS DataBase Reference | 1719-58-0(CAS DataBase Reference) | | NIST Chemistry Reference | Vinyldimethylchlorosilane(1719-58-0) | | EPA Substance Registry System | Silane, chloroethenyldimethyl- (1719-58-0) |
| | CHLORODIMETHYLVINYLSILANE Usage And Synthesis |
| Chemical Properties | clear light beige to amber liquid | | Physical properties | bp 82–83°C; d 0.874 gmL?1 at 25°C. | | Uses | Chlorodimethylvinylsilane is used to prepare silicon-containing polymers, silaheterocycles and new chelating ligands. | | Uses | Metallacycloalkanones have been prepared
by the cyclic hydroboration of dialkenyl silanes. In order to obtain
one such dialkenyl silane, chlorodimethylvinylsilane was allylated
with allyl magnesium bromide (eq 4).The dialkenyl silane
was then hydroborated sequentially with thexylborane, followed
by treatment with potassium cyanide, trifluoroacetic anhydride,
and finally hydrogen peroxide in sodium hydroxide to produce
the silacycloheptanone (eq 4).Cyclic silylketones were then
further reacted to form acyclic compounds with chiral centers that
are difficult to introduce without the use of the silicon tether.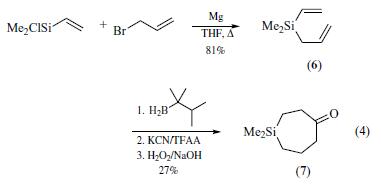 | | General Description | Chloro(dimethyl)vinylsilane (Dimethylvinylchlorosilane, Vinyldimethylchlorosilane, C4H9ClSi) is an organosilicon compound. It participates in the preparation of 1,1,2,2-tetramethyl-1,2-divinyldisilane. It undergoes [2+4] cycloaddition reaction with t-butyllithium in the presence of 2,3-dimethyl-1,3-butadiene to afford cycloadducts. | | Flammability and Explosibility | Highly flammable |
| | CHLORODIMETHYLVINYLSILANE Preparation Products And Raw materials |
|