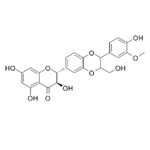
- Isosilybin
-

- $30.00 / 1mg
-
2024-11-12
- CAS:72581-71-6
- Min. Order:
- Purity: 98%
- Supply Ability: 10g
- Isosilybin
-
- $0.00 / 25kg
-
2024-03-28
- CAS:72581-71-6
- Min. Order: 25kg
- Purity: 30% 50%(HPLC) 80%(UV)
- Supply Ability: Inquiry
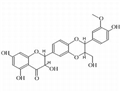
- Isosilybin
-
- $0.00 / 20mg
-
2023-02-24
- CAS:72581-71-6
- Min. Order: 20mg
- Purity: ≥98%(HPLC)
- Supply Ability: 100 g
|
| Product Name: | Isosilybin | | Synonyms: | ISOSILIBININ;ISOSILYBIN(PRIMARY STANDARD);Isosilybinin;(2R,3R)-2-[2,3-Dihydro-2-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-1,4-benzodioxin-6-yl]-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one;ISOSILYBIN hplc;Isosilybin (A+B) mixture;-(2,3-Dihydro-2-(4-hydroxy-3-Methoxyphenyl)-3-(hydroxyMethyl)-1,4-benzodioxin-6-yl)-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one;2-(2,3-Dihydro-2-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-1,4-benzodioxin-6-yl)-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one | | CAS: | 72581-71-6 | | MF: | C25H22O10 | | MW: | 482.44 | | EINECS: | | | Product Categories: | chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;Miscellaneous Natural Products;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 72581-71-6.mol | 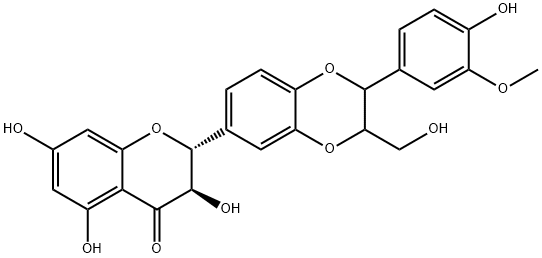 |
| | Isosilybin Chemical Properties |
| Boiling point | 793.0±60.0 °C(Predicted) | | density | 1.527±0.06 g/cm3(Predicted) | | storage temp. | Store at -20° C | | solubility | DMF: 20 mg/ml; DMF:PBS (pH 7.2) (1:9): 0.5 mg/ml; DMSO: 10 mg/ml; Ethanol: 0.1 mg/ml | | pka | 7.39±0.60(Predicted) | | form | Solid | | color | White-light yellow |
| | Isosilybin Usage And Synthesis |
| Structure | Only silybins and isosilybins contain the 1,4-dioxane ring system in their structure during Silymarin’s primary constituents. Silybin and isosilybin have the same trans conformation of C-2, C-3, C-7′ and C-8′. Silybin is considered the major and most active component of silymarin. | | Description | Isosilybin is a flavanolignan found in the extract of S. marianum fruits with antioxidant and anticancer activities. It inhibits lipid peroxidation in rat liver microsomes (IC50 = 32 μM) and reduces ADP/Fe3+-induced malondialdehyde (MDA) production and lactate dehydrogenase (LDH) release in rat hepatocytes. Isosilybin inhibits the production of reactive oxygen species (ROS), MDA and LDH release, and reduction in total antioxidant capacity induced by amyloid-β (25-35) (Aβ25-35) in HT-22 hippocampal cells. It also increases protein and mRNA expression of heme oxygenase-1 (HO-1), glutathione S-transferase (GST), and the aldo-keto reductases (AKCR) 1C1 and AKCR1C2 in HT-22 cells. In vivo, isosilybin (50 and 100 mg/kg) reduces tumor volume and increases tumor cell apoptosis in a DU145 prostate cancer mouse xenograft model. It also reduces expression of the tumor angiogenesis markers CD31, nestin, VEGF, VEGFR1, VEGFR2, phospho-Akt, and HIF-1α in tumor tissue without reducing blood vessel count in non-cancerous liver, lung, and kidney tissue in DU145 tumor-bearing mice. | | Uses | Isosilybin is useful in the treatment of liver disease | | Definition | The flavonolignan isosilybin is one of the components of the flavonoid complex extracted from the seeds of Silybum marianum (milk thistle), designated silymarin. Besides isosilybin, silymarin contains other flavonoids (congeners), such as silybin, 2,3-dehydrosilybin, silydianin, silychristin, isosilychristin, and taxifolin and ca. 20–30% is an undefined polymeric phenolic fraction. However, several recent studies have demonstrated that isosilybin is probably the most potent anticancer agent in silymarin. Isosilybin was shown to possess in vivo anti-proliferative, anti-angiogenic, pro-apoptotic, and cell-cycle modulatory properties. Moreover, isosilybin and especially isosilybin B inhibited the growth of advanced human prostate cancer cells in vivo without any toxic effects[1]. | | Definition | ChEBI: A flavonolignan isolated from Silybum marianum. | | References | [1] Radek Ga?ák . “Preparative method for isosilybin isolation based on enzymatic kinetic resolution of silymarin mixture.” Process Biochemistry 48 1 (2013): Pages 184-189.
|
| | Isosilybin Preparation Products And Raw materials |
|