- Amoxapine
-

- $64.00 / 500mg
-
2024-11-19
- CAS:14028-44-5
- Min. Order:
- Purity: 98.84%
- Supply Ability: 10g
- Amoxapine
-

- $15.00 / 1KG
-
2021-07-13
- CAS:14028-44-5
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Amoxapine
-

- $15.00 / 1KG
-
2021-07-10
- CAS:14028-44-5
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | Amoxapine Basic information |
| | Amoxapine Chemical Properties |
| Melting point | 175-1760C | | Boiling point | 469.9±55.0 °C(Predicted) | | density | 1.2613 (rough estimate) | | refractive index | 1.5800 (estimate) | | Fp | 9℃ | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | solubility | methanol: soluble | | form | Solid | | pka | pKa 7.6 (Uncertain) | | color | Crystals from benzene/pet ether | | Merck | 14,576 | | Stability: | Stable under recommended storage conditions., Stable Under Recommended Storage C | | CAS DataBase Reference | 14028-44-5(CAS DataBase Reference) | | NIST Chemistry Reference | Amoxapine(14028-44-5) |
| Hazard Codes | Xn | | Risk Statements | 22 | | Safety Statements | 36 | | RIDADR | 3249 | | WGK Germany | 3 | | RTECS | HQ4025500 | | HazardClass | 6.1(b) | | PackingGroup | III | | HS Code | 2934990002 | | Toxicity | LD50 in mice (mg/kg): 122 i.p.; 112 orally (Howell, 1972) |
| | Amoxapine Usage And Synthesis |
| Description | Amoxapine is a tetracyclic antidepressant with a wide range of pharmacological effects. It inhibits norepinephrine and serotonin reuptake, binding the respective transporters with Kd values of 16 and 58 nM. It has also been shown to act as either an antagonist or inverse agonist at serotonin 5-HT2A, 2B, 2C, 3, 6, 7 (Kis = 1 and 2 nM for 5-HT2A and 5-HT2C, respectively), dopamine D2, 3, 4 (Kd = 160 nM for D2), α1-adrenergic (Kd = 50 nM), and histamine H1 (Kd = 25 nM) receptors. | | Chemical Properties | Crystalline Solid | | Originator | Asendin,Lederle,US,1980 | | Uses | A tricyclic norepinephrine uptake inhibitor | | Uses | antidepressant, inhibits norepinephrine uptake | | Uses | Amoxapine is intended
more for relieving symptoms in patients with neurotic or situational depression. It has a
number of serious side effects. | | Definition | ChEBI: A dibenzooxazepine compound having a chloro substituent at the 2-position and a piperazin-1-yl group at the 11-position. | | Manufacturing Process | A mixture of 125 g of o-(p-chlorophenoxy)aniline hydrochloride and 100 ml of
dry pyridine is treated cautiously with a solution of 90 ml of ethyl
chlorocarbonate in 150 ml of ether. The mixture is kept at room temperature
for 3 days, diluted with about 500 ml of water and extracted with 300 ml of
ether, The ethereal extract is washed with 300 ml of water, dried over calcium
chloride, filtered and concentrated. The resulting ethyl o-(pchlorophenoxy)
carbanilate is obtained in a viscous oil suitable for use in the
next step without further purification.
A solution of 70 g of ethyl o-(p-chlorophenoxy)carbanilate and 120 g of Ncarbethoxypiperazine
in 100 ml of benzene containing a little sodium
methoxide is heated on a steam bath for about 5 days. The solvent is
removed by distillation and the residue is triturated with water. The resulting
solid is dissolved in ether and dried over sodium sulfate. Filtration and
concentration then yields ethyl 4-[[o-(p-chlorophenoxy)phenyl]carbamoyl]-1-
piperazinecarboxylate, melting at 89°C to 91°C, and suitable for cyclization.
A mixture of 10 g of the above piperazine carboxylate ester, 8 g of
phosphorus pentoxide and 20 ml of phosphorus oxychloride is heated under
reflux for about 1 day, diluted with 100 ml each of chloroform and benzene
and quenched with 200 g of ice. The mixture is made basic with 10% sodium
hydroxide. The organic layer is isolated and extracted with 150 ml of dilute
hydrochloric acid. The product is precipitated from the aqueous layer by
addition of 10% sodium hydroxide, extracted with benzene and dried over
potassium carbonate. Recrystallization from benzene-petroleum ether gives 2-
chloro-11-(1-piperazinyl)dibenz[b,f][1,4]oxazepine which melts at 175°C to
176°C. | | Brand name | Asendin (Lederle). | | Therapeutic Function | Antidepressant | | General Description | Consideration of the structure of amoxapine, 2-chloro-11-(1-piperazinyl)dibenz-[b,f] [1,4]oxazepine (Asendin), reinforcesthe fact that many antidepressants are very closelyrelated to antipsychotics. Indeed, some, including amoxapine,have significant effects at D2 receptors. The Nmethyl–substituted relative of amoxapine is the antipsychoticloxapine (Loxitane). The 8-hydroxy metabolite ofamoxapine is reportedly active as an antidepressant and asa D2 receptor blocker. | | Mechanism of action | Additionally, it is the N-desmethyl metabolite
of the antipsychotic loxapine. Amoxapine differs structurally from the other secondary TCAs in that it has both
a nitrogen and an oxygen atom in its 7-membered central ring and a piperazinyl ring rather than a
propylamino side chain attached to the central ring.Amoxapine is a less potent inhibitor of neuronal NE reuptake compared with the other secondary TCAs, with a
mechanism of action similar to that of desipramine. | | Pharmacology | The antidepressant action of amoxapine is comparable to that of imipramine and amitripty�line. It exhibits antagonistic activity on dopamine (D2) receptors. | | Pharmacokinetics | Amoxapine shares the toxic potentials of the TCAs, and
the usual precautions of TCA administration should be observed. Amoxapine resembles the atypical antipsychotic drugs in its intermediate affinity as an antagonist of dopamine-2 and of 5-HT2 receptors.
Amoxapine is rapidly and almost completely absorbed from the gastrointestinal (GI) tract. Amoxapine and its 8-hydroxyamoxapine metabolite have been detected in human milk at concentrations below steady-state therapeutic concentrations. | | Clinical Use | Amoxapine is a dibenzoxazepine TCA with antidepressant and antipsychotic effects that has shown
therapeutic effectiveness in patients with delusional depression. | | Safety Profile | Poison by ingestion andintraperitoneal routes. Human systemic effects byingestion: acute renal failure, acute tubular necrosis, BPlowering, coma, convulsions, decreased body temperature,EKG changes, excitement, fasciculations, heart ratechanges, hype | | Synthesis | Amoxapine, 2-chloro-11-(1-piperazinyl)-dibenz[b,f]oxazepine (7.3.2), is a
direct analog of the neuroleptic loxapine (6.5.3), differing only in the absence of a methyl
group in the piperazine region of the molecule. On the other hand, it could be included in
the class of tricyclic antidepressants, the main difference being the presence of a side chain
on the central 7-membered ring of the tricyclic system. Amoxapine, like loxapine, is syn�thesized from 2-(4-chlorobenzoxy)aniline, which as in loxapine synthesis is acidified with
chlorocarbonic acid into (6.5.1) and further transformed into ureide (7.3.1) upon reaction
with 1-carboethoxypiperazine. Cyclization by a mixture of phosphorous pentoxide and
phosphorous oxychloride into the dibenzoxazepine and subsequent alkaline hydrolysis
gives amoxapine (7.3.2) [50¨C53]. 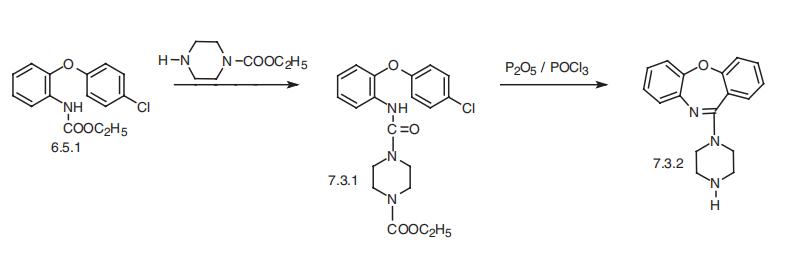
| | Metabolism | Amoxapine has the shortest elimination
time (~8 hours) of the secondary TCAs. It is metabolized in the liver principally to 8-hydroxyamoxapine and to
7-hydroxyamoxapine. Both of these metabolites are pharmacologically active and have half-lives of 30 and 6.5
hours, respectively. The hydroxylation of amoxapine is inhibited by ketoconazole, suggesting the involvement
of CYP3A4." |
| | Amoxapine Preparation Products And Raw materials |
|